RORγt is a transcription factor of the RAR-related orphan nuclear receptor (ROR) family. It often expresses in Th17 cells and plays an important role in TH17 cell differentiation.
RORγt can control the expression of a number of genes in Th17 cells. Key Th17 genes contain IL-17A, IL-17F, IL-23R, CCL20, and CCR6.
RORγt has a highly conserved across the NR family: hydrophobic ligand-binding pocket located within a ligand-binding domain (LBD). However, the transcriptional activity of RORγt is not dependent on ligand binding. RORγt is not recognized endogenous ligand, but it is sensitive to the binding of naturally occurring cholesterol derivatives.
NR orthosteric ligand binding pockets are the target for numerous and highly effective drug molecules.
numerous and highly effective drug molecules target NR orthosteric ligand binding pockets. However, the conserved area leads to issues associated with selectivity and mutation-induced resistance.
In addition, the dose level of most drugs has to compete with endogenous ligands.
As a result, it is extremely valuable to find out a small molecular targeting allosteric binding sites on NRs. This kind of compound can circumvent the above problems.
In this article, we will introduce an allosteric retinoic acid receptor-related orphan receptor γt (RORγt) inverse agonist, FM26.
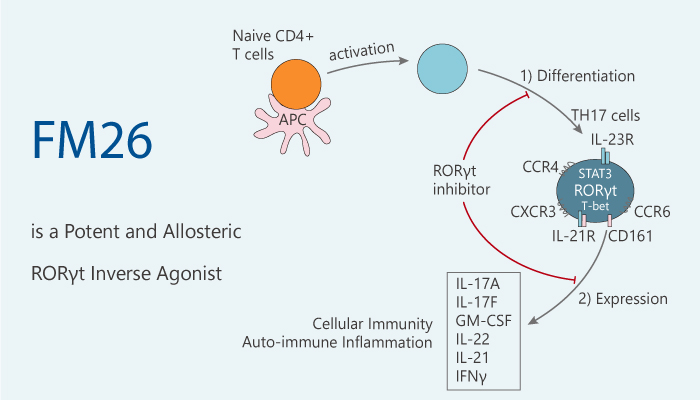
FM26 has a distinct isoxazole chemotype and exhibits an IC50 of 264 nM.
Because of RORγt promotion applies in IL-17a production, Measuring the reduction in IL-17a mRNA expression is a useful way to prove the activity of RORγt inverse agonist FM26.
EL4 is a murine lymphoblast cell line that constitutively expresses RORγt.
Before the measurement, FM26 treats EL4 cells for 24 hours. FM26 significantly reduces IL-17a mRNA expression 27-fold when it compares to the control group.
This result proves that the allosteric modulation of RORγt contributes to a cellular response. In addition, this kind of modulation is beneficial for the treatment of autoimmune disease.
Reference:
Meijer FA, et al.J Med Chem. 2020 Jan 9;63(1):241-259.