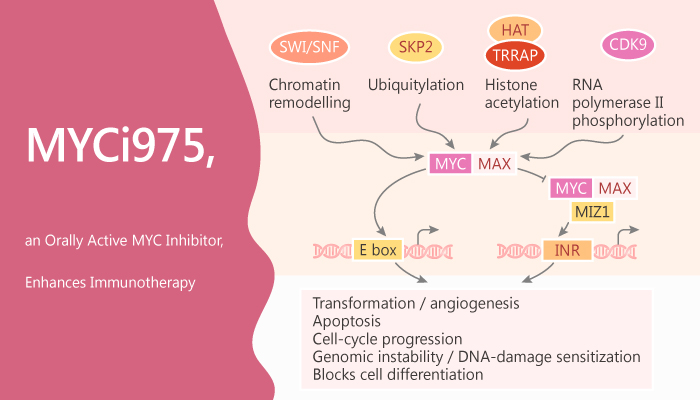
MYC proteins include MYC (also known as c-MYC), MYCL, and MYCN. They play critical roles in tumorigenesis and therapeutic resistance. MYC proteins are implicated in up to 70% of all human cancers via gene amplification, translocation, mRNA upregulation, and protein stabilization. MYC heterodimerizes with MAX to bind to a consensus sequence DNA element, enhancer box (E-box). Furthermore, it regulates downstream target genes primarily involved in proliferation, differentiation, cell-cycle progression, metabolism, apoptosis, and angiogenesis. Silencing MYC expression in multiple tumor models leads to tumor regression. MYC is an attractive cancer therapeutic target. In this study, MYCi975 is an orally active MYC inhibitor. It disrupts MYC/MAX interaction, promotes MYC T58 phosphorylation and MYC degradation, and impairs MYC driven gene expression.
MYCi975 is a close analog of 361 with improved therapeutic index. In vitro, MYCi975 inhibits cell viability in an MYC-dependent manner and selectively suppressed E-box luciferase activity. Furthermore, MYCi975 promotes MYC T58 phosphorylation and MYC degradation. In vivo, it also exhibits excellent pharmacokinetic profiles following p.o., i.p., or i.v. administration. The half-lives are 7 and 12 h when dosed at 100 and 250 mg/kg p.o., respectively. The Cmax values are 74 μM and 96 μM, respectively. In addition, MYCi975 treatment inhibits MycCaP tumors grown in immunocompetent FVB mice more strongly than in immunodeficient NSG mice, indicating that full anti-tumor efficacy of MYCi975 is also dependent on an intact immune system. MYCi975 also significantly increases survival in the MycCaP allograft model with animals tolerating a 100 mg/kg/day i.p. dosing for 14 days. Moreover, MYCi975 synergizes with anti-PD1 immunotherapy.
All in all, MYCi975 shows significant in vivo anti-tumor efficacy and better tolerability. It could be a promising starting point for the development of MYC inhibitor therapeutics.
Reference:
Han H, et al. Cancer Cell. 2019 Nov 11;36(5):483-497.e15.