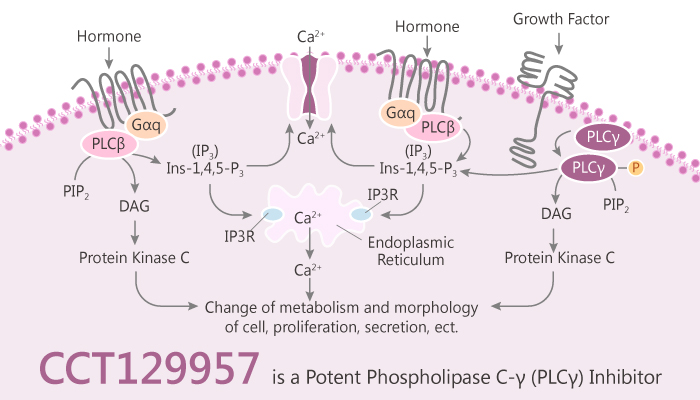
Phospholipase C (PLC) family members constitute a family of diverse enzymes. The PLC family members contain two highly related structural domains with conserved amino acid sequences. Phospholipase C-γ (PLC-γ) acts as a possible biological target for anti-cancer drug therapy. Furthermore, CCT129957 is a potent PLC-γ Inhibitor.
The PLC-γ subfamily divides into PLC-γ1 and the minor isoform PLC-γ2. The PLC-γ1 and 2 enzymes are plausible anticancer targets in cell motility important to invasion and dissemination of tumor cells. Phosphoinositide specific-phospholipase C (pi-PLC) is a membrane-bound protein that hydrolyzes PIP2 to DAG and IP3. Moreover, DAG activates the phospholipid-dependent serine/threonine kinase, PCK, and IP3 promotes the release of Ca2+ from intracellular stores. Motility of tumor cells is important for their invasion and dissemination leading to morbidity and death.
Activation of PLC-γ is an early event in pathways stimulated via receptor tyrosine kinases (RTKs). These pathways implicated in tumorigenesis making PLC-γ a plausible target for anticancer therapy. In addition, inhibition of PLC-γ1 activation blocks glioma cell motility and invasion of fetal rat brain aggregates. The active compound CCT129957 does not only inhibit the PLC-γ2 isoform but other PLCs as well due to their conserved binding site. Therefore, researchers performed virtual high throughput screening to identify active compounds.
Researchers tested twenty-six of the compounds in the calcium release cell-based assay. In particular, the most potent molecule is CCT129957 of the indole family with a GC50 of ∼15 μM. Especially, CCT129957 has IC50 ∼3 μM and Ca2+ release inhibition in squamous carcinoma cells of ∼15 μM.
All in all, CCT129957 is a PLC-γ2 inhibitor, which effects on the growth of human tumor cells.