Prolyl hydroxylases (PHDs) target hypoxia-inducible factor-1α (HIF-1α) for degradation. Hypoxia inactivates PHDs, causing accumulation of HIF-1α. In turn, HIF-1 further transactivates PHDs.
Asparaginyl hydroxylation also regulates HIFα. Asparaginyl hydroxylation is a modification that blocks the recruitment of the transcriptional co-activators CBP/p300 to HIFα. As a result, this effect causes reduced HIF transcriptional activity. In this study, Mun Chiang Chan et al identify IOX4. IOX4 is a potent and selective inhibitor of PHD2 in vitro. In particular, IOX4 potently inhibits PHD2 with an IC50 value of 1.6 nM using an antibody-based in vitro hydroxylation assay for PHD2 catalysis. In addition, IOX4 induces HIFα in cells and in wildtype mice with marked induction in the brain tissue.
IOX4 blocks prolyl-hydroxylation of HIF1α at both NODD (HyPro402) and CODD (HyPro564). On the contrary, IOX4 does not markedly affect the levels of PHD2. In von Hippel-Lindau (VHL)-competent HeLa cells, IOX4 induces HIF1α, with IOX4 being substantially more potent (activity being observed at ≥ 1 μM). Moreover, IOX4 induces HIF1α levels with EC50 values of 11.7 μM, 11.1 μM and 5.6 μM in MCF-7, Hep3B, and U2OS cells, respectively. IOX4 is a substantially more potent PHD inhibitor than IOX2.
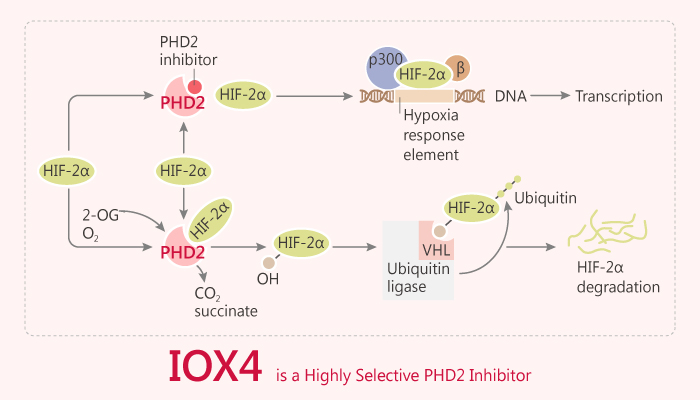
IOX4 as a highly potent and selective inhibitor of human PHD2. Importantly, IOX4 is effective at inducing HIFα in the mouse brain. IOX4 has an improved blood-brain barrier penetration compared to IOX2.
All in all, IOX4 is a potent and selective inhibitor of the hypoxia-inducible factor prolyl-hydroxylases with activity in the murine brain. IOX4 is useful for studies aimed at validating the upregulation of HIF for the treatment of cerebral diseases including stroke.