Ovarian cancer is one of the leading causes of death in women with gynecological cancers in the United States. About 70% of ovarian cancer cases are diagnosed at a late stage and therefore, poorly treatable. Protein disulfide isomerase (PDI), a 57-kDa chaperone protein, locates in the endoplasmic reticulum (ER). Acting as a thiol oxidoreductase, PDI catalyzes the formation, breakage, and rearrangement of disulfide bonds and therefore, regulates oxidative protein folding as well as cell viability. Some studied suggest that ER stress and unfolded protein response can activate PDI expression. Increased PDI levels found in a variety of human cancers, including ovarian, prostate, and lung cancers as well as lymphoma, glioma, acute myeloid leukemia, and melanoma. Inhibition of PDI activity leads to apoptosis in cancer. Thus, PDI is a promising druggable target. PACMA 31 is an irreversible, orally active PDI inhibitor with an IC50 of 10 μM.
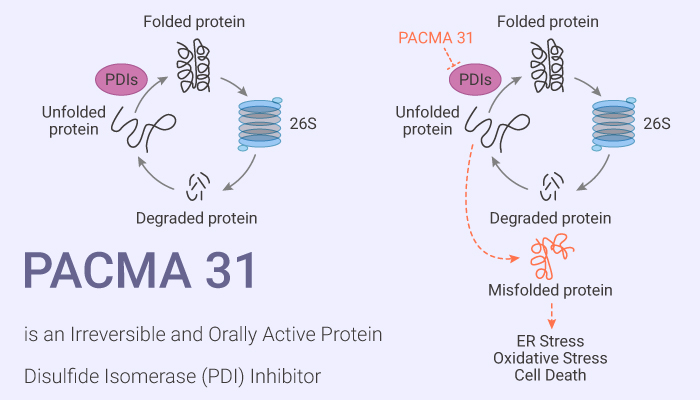
PACMA 31 is an irreversible, orally active PDI inhibitor. It forms a covalent bond with the active site cysteines of PDI. PACMA 31 also shows tumor targeting ability and significantly suppresses ovarian tumor growth without causing toxicity to normal tissues. Moreover, It significantly inhibits colony formation in OVCAR-8 cells in a dose-dependent manner. In vivo, PACMA 31 also shows tumor targeting ability and significantly suppressed ovarian tumor growth without causing toxicity to normal tissues.
In summary, PACMA 31 is an orally active PDI inhibitor with desirable pharmacological properties for cancer treatment. Furthermore, PDI is a druggable target for cancer therapy, and it opens a promising area of research to develop treatments with a unique mechanism of action.
Reference:
Xu S, et al. Proc Natl Acad Sci U S A. 2012;109(40):16348-16353.