Camptothecin is isolated from the bark of Camptotheca acuminita (a traditional Chinese medicine used for cancer treatment). Especially, Camptothecin is a topoisomerase I inhibitor. Furthermore, Camptothecin binds to the enzyme topoisomerase and DNA through hydrogen bonding and prevents DNA religation. In addition, this damages the DNA and causes apoptosis. Camptothecin exhibits a promising antitumor activity in various experimental tumors. In particular, T-2513 is an active camptothecin derivative.
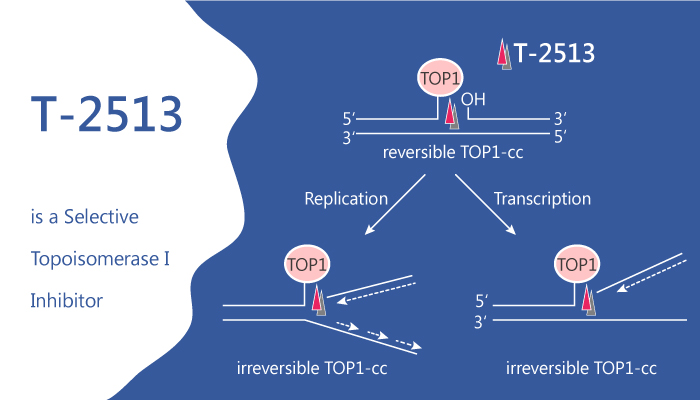
Delimotecan is cleaved to T-2513 by cathepsin B, which overexpresses in tumors, and further metabolizes to active metabolites, including SN-38. T-2513 is a selective inhibitor of topoisomerase I, responsible for the relaxation of torsionally strained supercoiled DNA. T-2513 binds covalently to and stabilizes the topoisomerase I–DNA complex and inhibits DNA replication and RNA synthesis, ultimately leading to cell death. The cytotoxic effects of T-2513 and metabolites are specific for the S-phase of the cell cycle.
T-2513 can metabolize to the CPT analogs, T-0055, T-1335, T-3921, and SN-38 (the active metabolite of CPT-11). Moreover, T-2513 has broad cytotoxicity against a range of human tumor cell lines. In the tumor, the sustained release of T-2513 occurs. In contrast, T-2513 disappears rapidly from the body. The significant increases in the amount and exposure time of released T-2513 in the tumor explain well the enhanced efficacy of T-0128. In conclusion, this study indicates that T-0128 improves the potency of T-2513, demonstrating the proof of the above concept.
T-2513 interacts directly with the DNA-topoisomerase I cleavable complex and to produce S-phase arrest and impaired G0 to G1 transition. These results demonstrate that T-2513 has a mechanism of action similar to camptothecin.