Lysophosphatidic acid (LPA) is a phospholipid growth factor that stimulates proliferation, chemotaxis, cation currents, and K+ currents in retinal pigment epithelial (RPE) cells. In particular, LPA is an extracellular signaling lipid that regulates cell proliferation, survival, and motility of normal and cancer cells. TAK-615 acts as a negative allosteric modulator (NAM) of the LPA1 receptor. Moreover, TAK-615 binds the LPA1 receptor with high affinity.
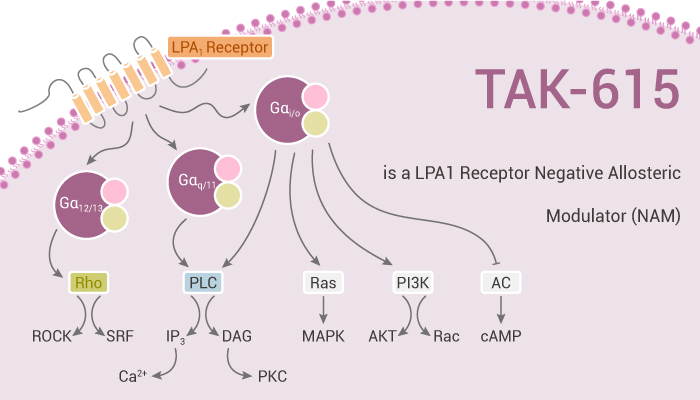
LPA is a small lysophospholipid molecule that activates multiple cellular functions through pathways with G-protein-coupled receptors. Six LPA receptors (LPAR1 to LPAR6) can connect to the downstream cell message-transmitting network. TAK-615 specifically binds to membranes expressing the human LPA1 receptor with an estimated Kd high affinity of 1.7 nM and Kd low affinity of 14.5 nM. TAK-615 is only able to partially inhibit the LPA response (~ 40% at 10 µM with an IC50 of 23 nM in the β-arrestin assay; 60% at 10 µM, IC50 of 91 nM in calcium mobilization assay).
LPA1 receptor links to the initiation and progression of a variety of poorly treated fibrotic conditions. Furthermore, TAK-615 acts as a negative allosteric modulator (NAM) of the LPA1 receptor. LPA describes a subset of small bioactive lysophospholipids that exert their biological effects through a family of six known G-protein coupled receptors (GPCRs). In addition, the LPA1 receptor functionally couples to Gαi, Gαq, and Gα12/13 signaling pathways and can activate β-arrestin.
All in all, LPA signaling plays a vital role in the normal physiological responses of many bodily processes. Surprisingly, TAK-615 acts as a negative allosteric modulator of the LPA1 receptor.