The cysteinyl leukotrienes (CysLTs) are inflammatory mediators closely associated with neuronal injury after brain ischemia through the activation of the CysLT1 receptor and the CysLT2 receptor. Specifically, CysLTs, namely leukotriene C4 (LTC4), LTD4, and leukotriene E4, are arachidonic acid-derived lipid mediators. Besides, CysLT1Rs is relevant to various inflammatory diseases such as bronchial asthma and allergic rhinitis. Moreover, CysLT2Rs increase vascular permeability and aggravate myocardial ischemia/reperfusion injury. Increased CysLTs induce central nervous system responses by activating their receptors. Furthermore, In the central nervous system, the production of CysLTs increases after ischemic injury in the rat brain, primary neurons, and astrocytes. CysLT1Rs and CysLT2Rs mediate acute ischemic neuronal injury and sequential microgliosis and astrocytosis in vivo. HAMI 3379 is a potent and selective Cysteinyl leukotriene (CysLT2) receptor antagonist with a protective effect on ischemic brain injury.
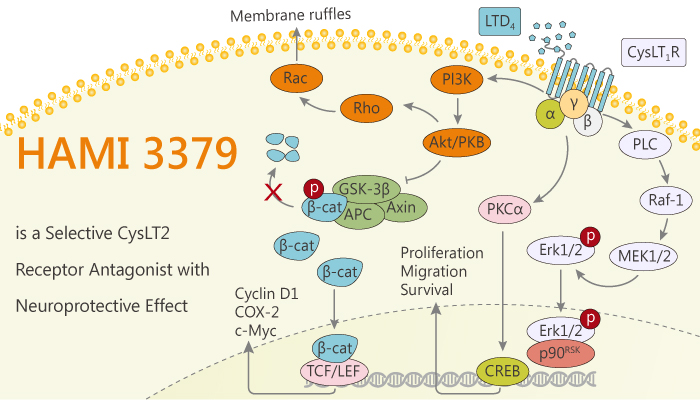
HAMI 3379 is a potent and selective Cysteinyl leukotriene (CysLT2) receptor antagonist. Meanwhile, HAMI 3379 has a protective effect on acute and subacute ischemic brain injury and attenuates microglia-related inflammation. Nonetheless, HAMI 3379 inhibited all of these responses, and its effects were the same as those of CysLT2R interference by CysLT2R short hairpin RNA, indicating CysLT2R dependence. Furthermore, HAMI 3379 effectively blocked CysLT2R-mediated microglial activation, thereby indirectly attenuating ischemic neuronal injury. In addition, intracerebroventricular injection of HAMI 3379 protects against acute brain injury in rats. Finally, HAMI 3379 has more potent protective effects than montelukast on ischemic neuronal injury in the relatively intact cellular environment. All in all, HAMI 3379 is a potent and selective CysLT2 receptor antagonist with a protective effect on ischemic brain injury and anti-inflammatory activity.
References:
Zhang XY, et al. J Pharmacol Exp Ther. 2013 Aug;346(2):328-41.