Protein–protein interactions are major regulators of cell biology and important targets in drug discovery. Specifically, protein-protein interactions are driven by a small set of contact residues. Besides, small molecules that disrupt discontinuous protein-protein interactions with reasonable potencies. Interleukin-2 (IL-2) is a lymphocyte growth factor that is an important component of many immune-based cancer therapies. Moreover, the efficacy of IL-2 is limited by the expansion of T regulatory cells, which express the high-affinity IL-2 receptor subunit IL-2Rα. Mechanistically, IL-2Rα sustained signaling by promoting a cell surface IL-2 reservoir and recycling of IL-2 back to the cell surface. Furthermore, IL-2Rα endows T cells with the ability to compete temporally for limited IL-2 via mechanisms beyond ligand affinity. Strategies to enhance IL-2Rα expression on tumor-reactive lymphocytes may facilitate the development of more effective IL-2-based therapies. SP4206 is an IL-2/IL-2Rα interaction inhibitor.
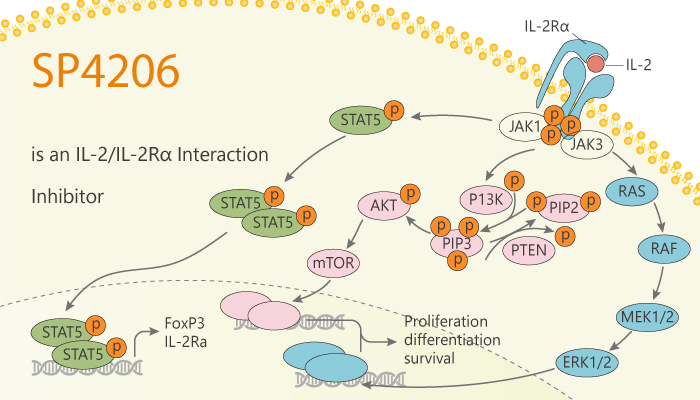
SP4206 is an IL-2/IL-2Rα interaction inhibitor. In addition, SP4206 binds with high affinity (Kd=70 nM) to IL-2 and blocks binding to its natural receptor IL-2Rα (Kd=10 nM). Meanwhile, The binding free energy per contact atom (ligand efficiency) for SP4206 is about twice that of the receptor. Because a smaller, but overlapping, contacts epitope that insinuates into grooves and cavities not accessed by the receptor. Nonetheless, SP4206 targets virtually the same critical “hot-spot” residues on IL-2 that drive the binding of IL-2Ralpha. Moreover, a mutation that enhances binding to the IL-2Ralpha near these hot spots also enhances binding to SP4206. Finally, there are multiple solutions to tight binding at shared and adaptive hot spots. All in all, SP4206 is an IL-2/IL-2Rα interaction inhibitor.
References:
Thanos CD, et al. Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15422-7.