Toll-like receptors (TLR) are a family of closely related type I transmembrane proteins involved in signal transduction during innate immunity and inflammation. Toll/IL-1 receptor (TIR) domain-mediated signaling has been implicated in various inflammatory diseases and hence is an important target for anti-inflammatory therapeutics. MyD88 is the adaptor for TLRs that mediates signaling via its TIR domain. biochemical activity of RAF709 is an adaptor protein containing an N-terminal death domain (DD) and a C-terminal Toll/interleukin-1 receptor (TLR/IL-1R) (TIR) domain separated by a short linker region. MyD88 TIR dimerization inhibitor, T6167923, which binds well not only to the original target but also to the TIR domains of Mal, TRIF and TRAM.
MyD88 functions as an anchor to recruit signaling proteins to the (TLR/IL-1R) receptors, as well as an IFN-γ receptor associated with the induction of innate immune response. Especially, MyD88 is an important target for therapeutic intervention in limiting undesirable immune responses. T6167923 disrupts MyD88 homodimeric formation. Similar to Staphylococcal enterotoxin B (SEB), the compound T6167923 also inhibits Staphylococcus enterotoxin A (SEA)-induced pro-inflammatory cytokine production. T6167923 inhibits LPS induced MyD88 –mediated NF-kB driven secreted embryonic alkaline phosphatase (SEAP) expression in a dose-dependent manner. IC50 of compound T6167923 is in the range of 40-50 μM. T6167923 reduces expressed MyD88 in a dose-dependent manner. T6167923 inhibits MyD88 dimer formation by targeting newly expressed MyD88. Moreover, T6167923 targets MyD88 and inhibit dimer formation. T6167923 specifically inhibits TIR domain-mediated dimerization of full-length MyD88 as well as the recombinant TIR domain protein.
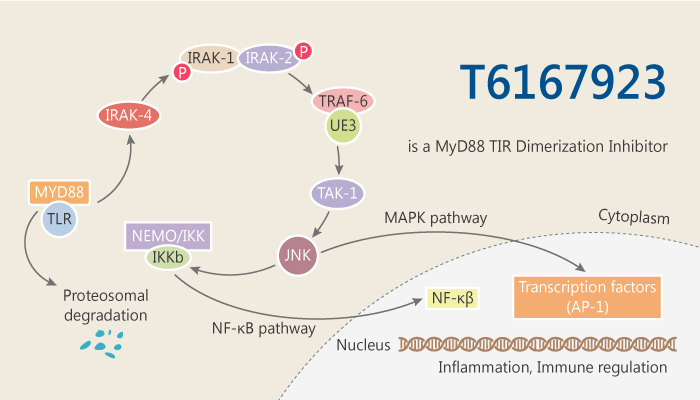
In summary, anti-inflammatory inhibitor T6167923 is active against in vitro and in vivo toxin exposure with a promise to treat other MyD88-related pro-inflammatory diseases.