Interferons (IFNs) are a group of signaling proteins in response to the presence of several viruses. Cyclic GMP-AMP Synthase (cGAS, cGAMP synthase) is a cytosolic DNA sensor. Especially, cGAS activates the type I interferon pathway. Crucially, cGAS belonging to the nucleotidyltransferase family, activates a type-I interferon response. In particular, cGAS is part of the cGAS-STING DNA sensing pathway. Moreover, cGAS binds to microbial DNA as well as self DNA that invades the cytoplasm, and catalyzes cGAMP synthesis. cGAMP then functions as a second messenger that binds to and activates the endoplasmic reticulum protein STING, thereby triggering type-I interferon production. In addition, cGAS has been shown to be an innate immune sensor of retroviruses including HIV. Cyclic GMP-AMP synthase (cGAS) initiates the innate immune system in response to cytosolic dsDNA. Cytosolic cGAS binds dsDNA and catalyzes the production of second messenger cGAMP. cGAMP then binds to stimulator of interferon genes (STING).
Recently, a number of other cytosolic DNA sensors exist, including Absent in Melanoma 2 (AIM2), DNA-dependent activator of IRFs (DAI) and IFN-γ-inducible protein 16 (IFI16) but accumulating evidence suggests cGAS is the primary sensor in innate immune activation.
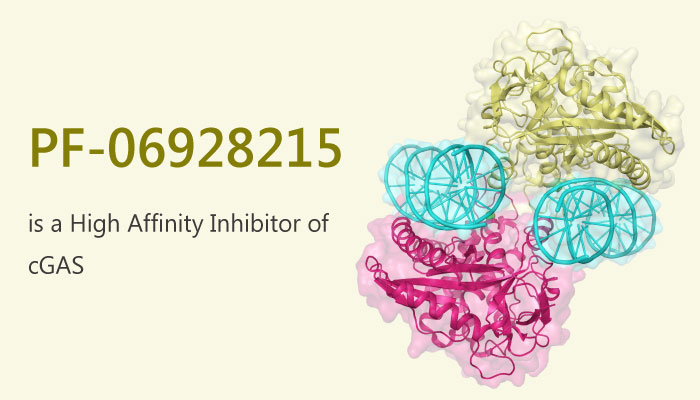
In this study, Justin Hall, et al reported the development of a high affinity inhibitor from a low affinity fragment hit with supporting biochemical and structural data showing these molecules bind to the cGAS active site. Justin Hall, et al also reported a new high throughput cGAS fluorescence polarization-based assay to enable the rapid identification and optimization of cGAS inhibitors.
Notably, PF-06928215 has the binding affinity of 200 nM with a corresponding increase in inhibitory potency in the cGAS assay; PF-06928215 inhibits cGAS activity with an IC50 value of 4.9 μM.