Cyclic AMP (cAMP) is a universal second messenger in cells of all biological systems. Specifically, cAMP homeostasis is affected by synthesis, interaction, and degradation, and is realized by the multigene family of cyclic nucleotide phosphodiesterase (PDEs). Besides, PDE is an enzyme that breaks the phosphodiester bond. Cyclic nucleotide phosphodiesterases contain a group of enzymes that degrade phosphodiester bonds in camp and cGMP. Moreover, they regulate the location, duration, and amplitude of cyclic nucleotide signaling in the subcellular domain. Therefore, PDE is an important regulator of signal transduction mediated by these second messenger molecules. cAMP PDE4 enzymes degrade cGMP and enhance the compartmentalization of cAMP signals by targeting specific protein complexes and intracellular regions. Furthermore, the cAMP-specific PDE4 family plays a key role in regulating various key physiological processes and supporting molecular pathology in various diseases. MR-L2 is a reversible and noncompetitive allosteric activator of long-isoform PDE4.
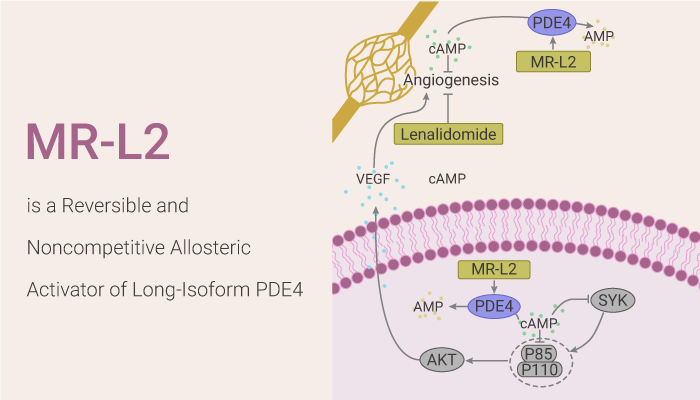
MR-L2 is a reversible and noncompetitive allosteric activator of long-isoform PDE4.
First of all, MR-L2 activates representative PDE4 long-isoform variants (PDE4A4, PDE4B1, PDE4C3, PDE4D5). MR-L2 suppresses PGE2-induced MDCK cell cyst formation with an EC50 of 1.2 µM.
Secondly, MR-L2 does not activate the exemplary short isomers of PDE4A, B, and D families. the stimulatory effects are specific to the PDE4 family and MR-L2 enhances the activity of exemplars from any of the other 10 families within the PDE superfamily. MR-L2 failed to activate short PDE4 isoforms, which lack the regulatory UCR1 domain and so are insensitive to activation by PKA.
Last but not the least, activation by MR-L2 requires the dimeric assembly of long-Form PDE4D5. MR-L2 elicits an allosteric activation of the PDE4D5 long isoform. MR-L2 reduces intracellular cAMP in MDCK cells without affecting PDE4 expression or cell viability.
All in all, MR-L2 is a reversible and noncompetitive allosteric activator of long-isoform PDE4.
References:
Omar F, et al. Proc Natl Acad Sci U S A. 2019 Jul 2;116(27):13320-13329.