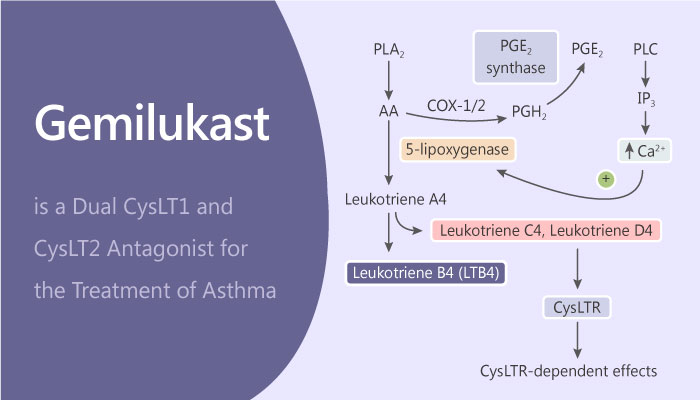
Asthma was introduced in previous blog “MK-8318, a Potent and Selective CRTh2 Receptor Antagonist in Asthma”, inhibiting CRTh2 Receptor is a potential therapy to treatment asthma. Moreover, there are many other allergic related targets have been found like Cysteinyl leukotrienes (CysLTs). CysLTs are known to induce airway smooth muscle constriction, enhance vascular permeability and recruit eosinophils, which contribute to asthma symptoms. Today, I would like to introduce a dual CysLT1 and CysLT2 antagonist Gemilukast (ONO-6950).
A study from Yasuo Yonetomi identified and verified the effect of Gemilukast (ONO-6950) both in vitro and in vivo.
As a result, in vitro, Gemilukast (ONO-6950) is an orally active and potent dual cysteinyl leukotriene 1 and 2 receptors (CysLT1 and CysLT2) antagonist. The IC50 values are 1.7, 25 nM for human CysLT1 and CysLT2, respectively. Both Gemilukast (ONO-6950) and montelukast inhibit human CysLT1 receptor-mediated calcium response with IC50 values of 1.7 and 0.46 nM, respectively.
Additionally, in vivo, Gemilukast (ONO-6950) at 0.03 to 10 mg/kg, p.o. dose-dependently attenuates LTC4-induced bronchoconstriction with almost complete inhibition at 3 mg/kg. The effect of Gemilukast is significantly stronger than that of montelukast at the dose of 1 mg/kg or more. Gemilukast (0.03 to 1 mg/kg, p.o.) also dose-dependently attenuates LTD4-induced airway vascular hyperpermeability with complete inhibition at 0.3 mg/kg. Furthermore, Gemilukast at 0.1 to 3 mg/kg, p.o. dose-dependently inhibits OVA-induced bronchoconstriction. The inhibitory effect of Gemilukast at 3 mg/kg is significantly greater than that of montelukast alone and comparable to that of combination therapy with montelukast and BayCysLT2RA
To conclude, Gemilukast is a dual CysLT1 and CysLT2 antagonist, which has potential to treat asthma.
Reference: