STING is a protein with four putative transmembrane domains and resides in the endoplasmic reticulum. STING widely expresses in both immune cells (including innate immune cells and adaptive immune cells) and non-immune cells. In particular, STING is a signalling molecule associated with the endoplasmic reticulum. Especially, STING is essential for controlling the transcription of numerous host defence genes, including type I interferons and pro-inflammatory cytokines. Activation of the STING protein by cyclic dinucleotide metabolites plays a critical role in antitumor immunity. STING links innate immunity to biological processes ranging from antitumor immunity to microbiome homeostasis. SR-717 is a non-nucleotide, small-molecule STING agonist.
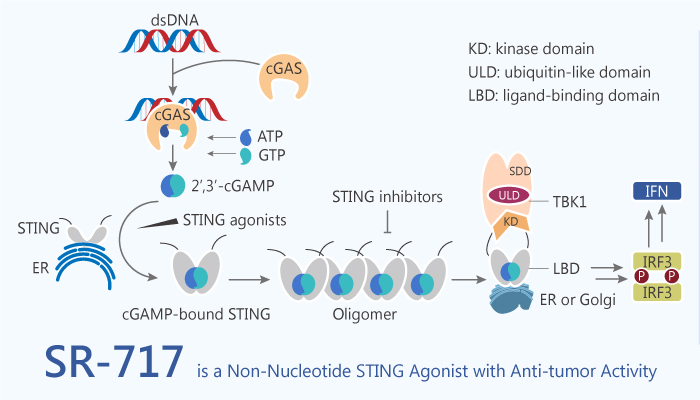
SR-717 displays antitumor activity, promotes the activation of CD8+ T, natural killer, and dendritic cells in relevant tissues; and facilitates antigen cross-priming. SR-717 also induces the expression of clinically relevant targets, including programmed cell death 1 ligand 1 (PD-L1), in a STING-dependent manner. Further assessment of T cell function revealed that SR-717 treatment significantly increased the frequency of granzyme B and CD107a+ CD8 T cells in both spleen and tumor.
Systemic SR-717 administration induces the activation of CD11c+ CD8α dendritic cells, and enhances cross-priming of CD8 T cells. Moreover, SR-717 induces the expression of PD-L1 in THP1 cells and in primary human peripheral blood mononuclear cells in a STING-dependent manner. STING activation with SR-717 induces the expected stimulatory events and elicites a corresponding induction of molecules known to suppress immune responses.
All in all, SR-717 is a stable cGAMP mimetic that activates STING by inducing the same closed conformation with antitumor immunity.