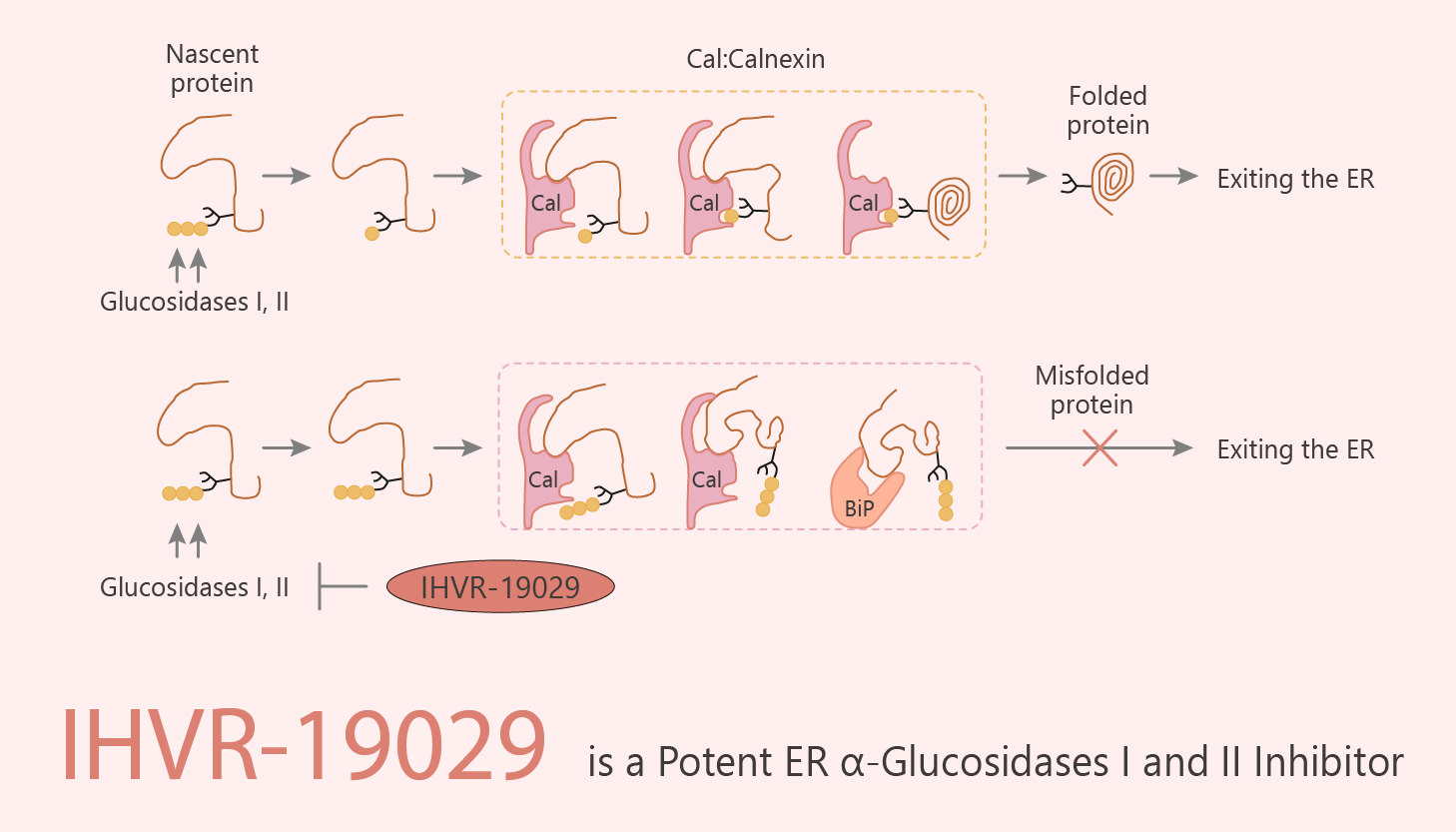
Host cellular endoplasmic reticulum (ER) α-glucosidases I and II are essential for the maturation of viral glycosylated envelope proteins. They use the calnexin mediated folding pathway. Inhibition of these glycan processing enzymes leads to the misfolding and degradation of these viral glycoproteins, thereby reduction in virion secretion. ER α-glucosidases I and II sequentially remove the three glucose residues from the high-mannose N-linked glycans attached to nascent glycoproteins. It is critical for the subsequent interaction between the glycoproteins and ER chaperones, calnexin, and calreticulin. In addition, due to the highly dynamic nature of the viral replication, it is conceivable that inhibition of ER α-glucosidases might differentially disturb the maturation and function of viral envelope glycoproteins. Thus, it inhibits viral particle assembly and/or secretion. In this study, IHVR-19029 is a potent endoplasmic reticulum (ER) α-glucosidases I and II inhibitor. It has an IC50 of 0.48 μM for ER a-glucosidase I.
IHVR-19029 efficiently inhibits Bovine viral diarrhea virus (BVDV), Tacaribe virus (TCRV), and Dengue virus (DENV) with EC50s of 0.25, 0.74, and 1.25 μM, respectively. Meanwhile, it also inhibits EBOV and MARV infection in mice. Moreover, the combination of suboptimal doses of IHVR-19029 and T-705 significantly increased the survival rate of infected animals. Therefore, a combination of IHVR-19029 with Favipiravir improves antiviral efficacy.
In summary, IHVR-19029 is a lead endoplasmic reticulum α-glucosidases I and II inhibitor, and it efficiently blocks the replication of several hemorrhagic fever viruses. Furthermore, it efficiently protects mice from lethal Ebola and Marburg virus. In addition, the Combination of IHVR-19029 and T-705 synergistically inhibits the replication of Yellow fever and Ebola viruses in cultured cells.
Reference:
Chang J, et al. Antiviral Res. 2013;98(3):432-440;
Ma J, et al. Antiviral Res. 2018 Feb;150:112-122.