The toll-like receptors (TLRs) play an essential role in host defense. TLRs initiate the activation of innate immune responses, and they via recognition of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). Most TLRs signal through the adapter molecule myeloid differentiation factor 88 (MyD88). Only TLR3 signals through a MyD88-independent pathway, and TLR4 signals through both MyD88 and TRIF. TLR5 expression is closely related to various infectious diseases caused by bacteria. A lack of TLR5 results in metabolic syndrome and altered gut microbiota, which may induce colitis, colitis, even adipose tissue inflammation. TLR5 can contribute to endophthalmitis pathogenesis due to Bacillus intraocular infection as well. It also responds to a variety of cancer progressions. TLR5 is also related to autoimmune diseases and virus infection according to recent studies. In this study, TH1020 is a potent and selective TLR5/flagellin complex antagonist, with an IC50 of 0.85 μM.
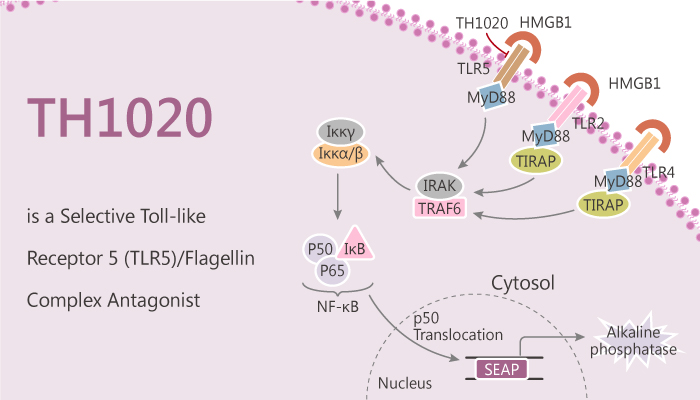
TH1020 at 0.78μM shows nearly negligible inhibition of TLR2, TLT4, TLR7, and TLR8, though it demonstrated weak inhibition of TLR3. TH1020 binds to the interface of the two copies of TLR5 and disrupts the tetrameric complex formation. In addition, it almost completely abolishes the TLR5-mediated TNF-α secretion at 0.37 μM. Therefore, TH1020 suppresses the downstream signaling of TLR5 in a consistent manner as it targets the TLR5/flagellin complex formation.
In summary, TH1020 is a potent and selective TLR5/flagellin complex antagonist and inhibits flagellin-induced TLR5 signaling. It provides a much-needed molecular probe for studying this important protein-protein interaction. Ultimately, it also could lead to potential pharmaceutical candidates for the treatment of TLR5-related inflammatory diseases.
Reference:
Lei Yan, et al. ChemMedChem. 2016 Apr 19;11(8):822-6.