CXCR4 is a chemokine receptor specific to matrix derived factor-1 (SDF-1), which has effective chemotactic activity on lymphocytes. Specifically, CXCR4 is one of several chemokine co receptors that HIV can use to infect CD4 + T cells. Chemokine/receptor pairs, SDF-1/CXCR4 are candidate promoters for breast cancer metastasis. Besides, the overexpression of CXCR4 in 67% of breast cancer samples indicates that the expression of CXCR4 is increased, and the prognosis of CXCR4 is poor. Therefore, inhibition of the CXCR4 activity may hinder the transfer process. In some cancers, CXCR4 expression on malignant cells in primary human tumors is relevant to lymph node metastasis. Moreover, CXCR4 ligand CXCL12 is in the common metastasis sites of these malignant tumors. Furthermore, we have found that blocking CXCR4 activity can inhibit the growth of primary tumors and metastasis of the xenograft model. CTCE-9908 is a potent and selective CXCR4 antagonist.
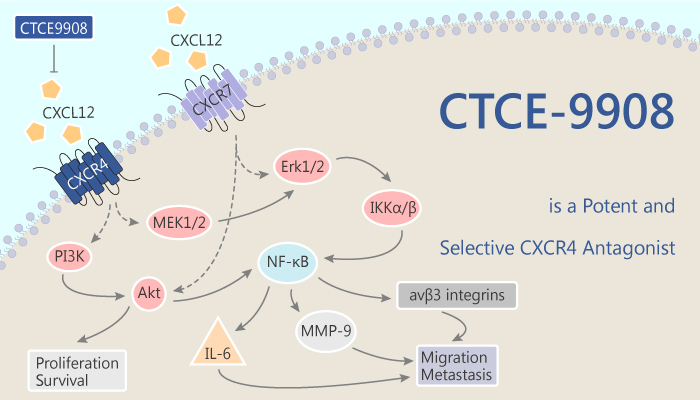
CTCE-9908 is a potent and selective CXCR4 antagonist.s
How does CTCE-9908 work on the target? Let’s study it together. In the beginning, CTCE-9908 induces mitotic catastrophe, inhibits migration, and induces cytotoxicity in CXCR4-expressing ovarian cancer cells.
In the second place, CTCE-9908 inhibits migration and growth in CXCR4-expressing in ovarian cancer cell lines (IGROV, TOV21G, and SKOV3). Meanwhile, CTCE-9908 inhibits ovarian cancer cell migration to CXCL12. Nonetheless, CTCE-9908 does not cause apoptosis or cellular senescence but induces multinucleation, G2-M arrest, and abnormal mitosis in ovarian cancer cells. Nonetheless, CTCE-9908 deregulates DNA damage checkpoint proteins and spindle assembly checkpoint proteins at G2-M phases of the cell cycle.
Last but not the least, CTCE-9908 alone slows the rate of primary breast tumor growth. Importantly, CTCE-9908 has a 45% inhibition of primary tumor growth at 3.5 weeks of treatment with 50 mg/kg in FVB/N TgN (MMTV-PyMT)634 male mice.
All in all, CTCE-9908 is a potent and selective CXCR4 antagonist.
References:
Joseph Kwong, et al. Mol Cancer Ther. 2009 Jul;8(7):1893-905.