Psoriatic arthritis (PsA) is a complex and heterogeneous inflammatory disease that affects 20% to 30% of patients. Pro-inflammatory cytokines are the potential targets in inflammatory diseases. They have led to the creation of a number of anti-cytokine monoclonal antibodies. However, dual inhibitor antibodies target two different cytokines simultaneously potentially offering a better disease control.
In recent studies, IL-17F is also increased in psoriatic skin and synovial cell in PsA. It supports the rationale for targeting both IL-17A and IL-17F in psoriatic disease. Bimekizumab is the first-in-class monoclonal antibody to simultaneously target IL-17A and IL-17F. On October 17, 2023, the U.S. Food and Drug Administration approved Bimekizumab to treat moderate to severe plaque psoriasis in adults.
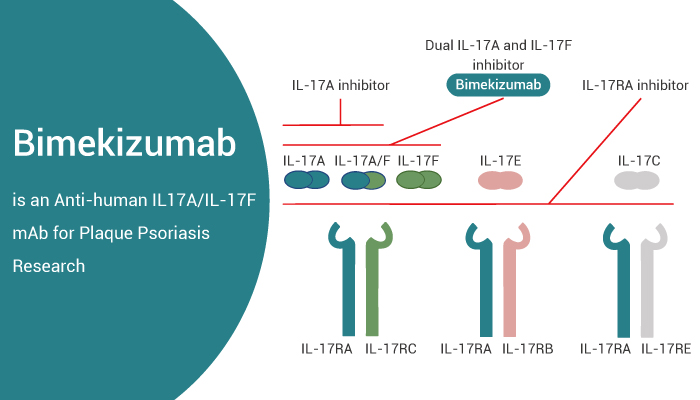
Bimekizumab is the first dual inhibitor of IL-17A and IL-17F for the research of psoriatic arthritis.
Bimekizumab is a humanized monoclonal IgG1 antibody that selectively neutralizes both IL-17A and IL-17F. On the one hand, Bimekizumab blocks the inflammation-driven osteogenic differentiation. Meanwhile, Bimekizumab (10 μg/mL; 1 h) suppresses osteocommitment of hPDCs by AS serum. On the other hand, in vitro models, Bimekizumab appears to be as potent as Ixekizumab at inhibiting IL-17A (also more potent than Secukinumab).
But, unlike those drugs, it also possesses the unique ability to inhibit IL-17F as well, functioning as a dual inhibitor. Bimekizumab demonstrates dose-proportional linear pharmacokinetics, with a half-life ranging from 17 to 26 days. In addition, Unlike Brodalumab, Brodalumab is an IL-17 receptor A blocker. It can target not only IL-17A and F signaling but also IL-17 C, D and E. Bimekizumab spares IL-17E (also known as IL-25), for example, it has anti-inflammatory properties.
In a word, Bimekizumab is a dual IL-17A/F inhibitor. It can be used for the research of psoriatic arthritis, and ankylosing spondylitis.
Reference:
[1] Shah M, et al. RMD Open. 2020 Jul;6(2):e001306.
[2] Oliveira DG, et al. 2021 Mar 9;15:1045-1053.