The curtain has fallen on 2023, with 2024 ushering in a new chapter! Looking back at 2023, the momentum of FDA new drug approvals was as hot as ever. The total number of new drugs approved by the FDA in 2023 hit a five-year high! The Center for Drug Evaluation and Research (CDER) approved 55 innovative drugs. The Center for Biologics Evaluation and Research (CBER) approved 7 cell and gene therapy products. This includes Casgevy, which is the world’s first approved CRISPR gene editing therapy. Two cell therapy products are also included. Lastly, Vowst, the first oral fecal microbiota product, is a part of this as well.
Cell and Gene Therapy (CGT) drugs refer to the use of biotechnology methods to modify individual gene expression or repair abnormal genes through gene addition, gene modification, gene silencing, etc., in order to achieve precise cure of diseases. Specific drug forms include gene editing technology, stem cell therapy, and CAR-T cell therapy.
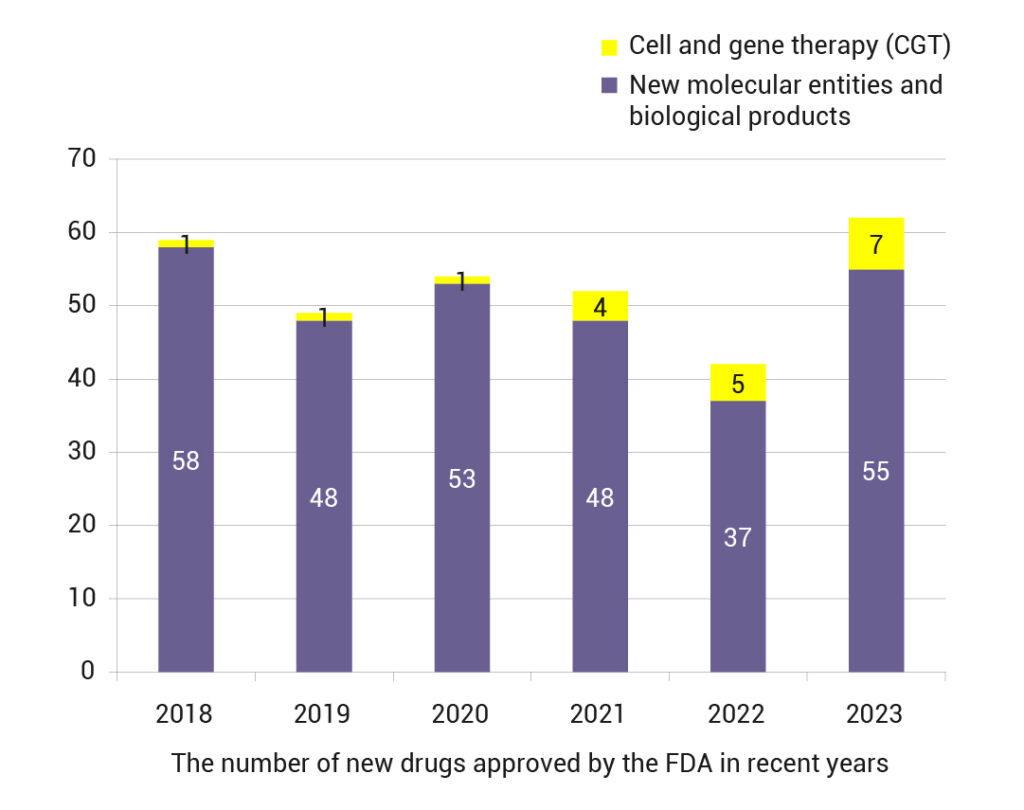
In recent years, the number of approvals for CGT new drugs has been increasing year by year. In the past 2023, the FDA has successively approved 11 CGT drugs, of which 7 were first approved, accounting for 11.3% of the new drugs approved in 2023.
There are some GMP small molecules in the field of CGT, which can be used for stem cell reprogramming, induction of differentiation, proliferation and expansion, stemness maintenance, and transdifferentiation. Its functions include activating and alleviating CAR-T depletion, helping you develop CGT therapies.
