Core binding factor (CBF) leukemias account for ∼24% of adult acute myeloid leukemia (AML) and 25% of pediatric acute lymphocytic leukemia (ALL). CBF leukemias show translocations or inversions that affect transcription factor genes RUNX1 or CBFB. Transcription factors RUNX1 and CBFβ form a heterodimer for DNA binding and regulation of gene expression. Genes encoding both proteins play key roles in hematopoiesis. They are involved in leukemogenesis through recurrent chromosome abnormalities, such as a chromosome 16 inversion. It generates a fusion gene between CBFB and MYH11 in acute myeloid leukemia (AML) subtype M4Eo. However, the physical interactions between RUNX1 fusion proteins (RUNX1-ETO and TEL-RUNX1) and CBFβ, and between the CBFβ fusion protein (CBFβ-SMMHC) and RUNX1 are critical for the pathogenesis of CBF leukemias. In this study, Ro5-3335 acts as an inhibitor of CBF leukemia.
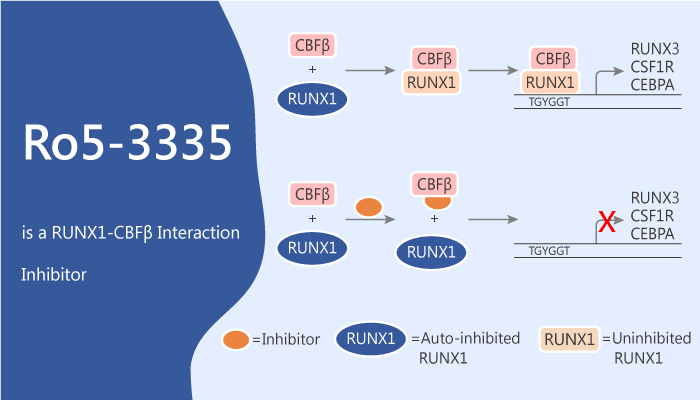
Ro5-3335 is a RUNX1-CBFβ interaction inhibitor that represses RUNX1/CBFB-dependent transactivation.
The authors develop high-throughput AlphaScreen and time-resolved fluorescence resonance energy transfer (TR-FRET) methods to quantify the RUNX1-CBFβ interaction. Ro5-3335 is a benzodiazepine. It is able to interact with RUNX1 and CBFβ directly, repress RUNX1/CBFB-dependent transactivation in reporter assays and repress runx1-dependent hematopoiesis in zebrafish embryos. Meanwhile, Ro5-3335 has antiproliferative activity against human CBF leukemia cell lines. It shows IC50s of 1.1 μM, 21.7 μM, and 17.3 μM for ME-1, Kasumi-1, and REH, respectively. In addition, it inhibits definitive hematopoiesis in zebrafish embryos. Moreover, Ro5-3335 does not completely break apart RUNX1-CBFβ interaction, but changes the conformation of their complex or increases the distance between RUNX1 and CBFβ in the complex. Furthermore, Ro5-3335 rescues the preleukemic phenotype in a RUNX1-ETO transgenic zebrafish. In addition, Ro5-3335 reduces the leukemia burden in a mouse CBFB-MYH11 leukemia model.
In summary, Ro5-3335 is a promising compound that will lead to a unique for CBF leukemias.
Reference:
Cunningham L, et al. Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14592-7.