The ubiquitin-proteasome system exerts numerous essential regulatory functions in almost all cell biological pathways. The 26S proteasome degrades polyubiquitylated protein substrates and consists of a 19S regulator bearing ubiquitin receptors and an ATPase ring in charge of protein unfolding as well as a 20S proteolytic core complex. Proteasome inhibitors are applied in anti-cancer therapy, especially for multiple myeloma and relapsed mantle cell lymphoma. To improve pharmacological and toxicological profiles, scientists dedicated their lives to develop new proteasome inhibitors. Up to now, PR-957 (later renamed ONX 0914) represents a selective for LMP7 in a concentration-dependent manner. Besides, ONX 0914 represents the prototype LMP7-selective inhibitor in many studies. Additionally, a study from Michael Basler discovered and identified a novel, potent, specific and cell-permeable proteasome β1i (LMP2) subunit inhibitor, ML604440.
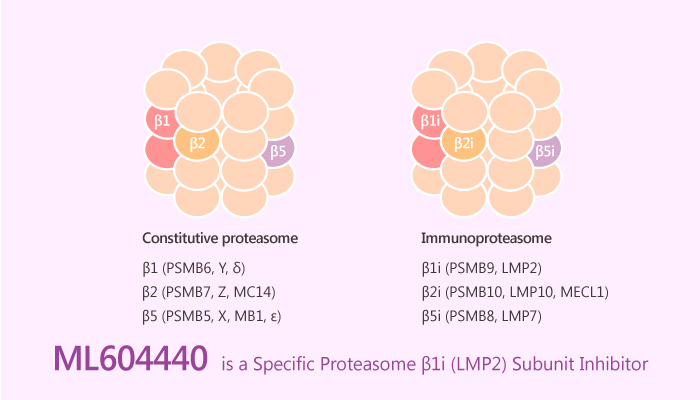
ML604440 impairs MHC class I cell surface expression, IL-6 secretion, and differentiation of naïve T helper cells to T helper 17 cells. In addition, ML604440 strongly ameliorates disease in experimental colitis and EAE. Hence, the co-inhibition of LMP2 and LMP7 appears to be synergistic and advantageous for the treatment of autoimmune diseases. ML604440 is a β1i inhibitor, did not induce PARP1 cleavage but potentiated the effect of PR957.
However, preclinical data is still lacked and more work needs to be carried out. Collectively, ML604440 is a promising clinical candidate to treat autoimmune diseases.
Reference:
Basler M, et al. EMBO Rep. 2018 Dec;19(12). pii: e46512.