A robust antibody response is essential for efficient identification and eradication of pathogenic microbes and toxins. Dysregulation of the antibody response can lead to autoimmune disease. Specific antigen encounter by B lymphocytes induces clonal expansion that encompasses several distinct stages of differentiation. Antibody-producing or long-lived memory B cells is of great importance for understanding vaccine efficacy, and how/where B cell differentiation may go awry to cause autoimmunity. In this context, there is a growing appreciation of microenvironment-specific influences on B cell differentiation within the lymphoid tissue. B cells express Epstein-Barr virus-induced gene 2 (EBI2), and the activation highly induces it. Recent experiments reveal that EBI2-/- B cells exhibit defective migration, resulting in strongly impaired T cell-dependent antibody responses. Here, the study describes a potent functional antagonist of EBI-2, ML401, and is also a potent chemical probe.
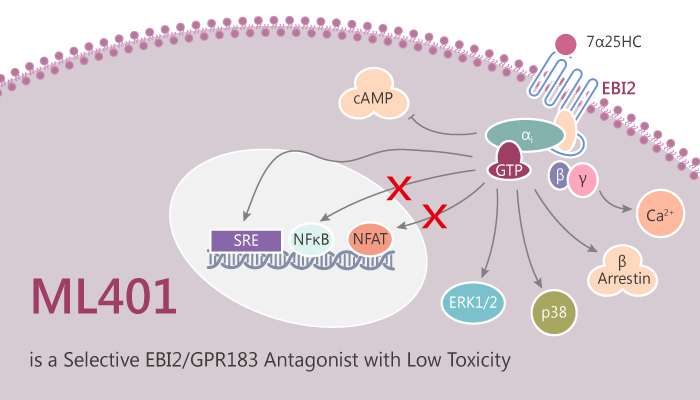
ML401, as a chemical probe, selectively antagonizes EBI-2 with an IC50 of ~ 1 nM. Furthermore, ML401 also displays activity in a chemotaxis assay with an IC50 of ∼6 nM). Besides, ML401 shows no toxicity towards immortalized Fa2-N4 human hepatocytes and non-cytotoxic in both LnCap and IMR-32 cells. In addition, ML401 has a clean profile in a Eurofins/Ricerca panel as well as excellent rodent pharmacokinetics.
ML401 exhibits excellent permeability across the pH range of the donor compartment. It shows very good stability in both human plasma and mouse plasma. In addition, ML401 shows moderate stability in both human and mouse liver microsomes after 1 hour.
In summary, ML401 is a potent chemical probe and functional antagonist of EBI-2. It should be a valuable tool to explore the in vivo effects of EBI-2 inhibition.
Reference:
Ardecky R, et al. Probe Reports from the NIH Molecular Libraries Program [Internet].