 The abelson (ABL) family of nonreceptor tyrosine kinases, including ABL1 and ABL2, transduce diverse extracellular signals to protein networks. Recently, Morita et al. showed that the non-receptor ABL tyrosine kinases enhanced the enzymatic activities of the endoplasmic reticulum (ER) transmembrane kinase, IRE1a, thereby potentiating ER stress-induced apoptosis.
The abelson (ABL) family of nonreceptor tyrosine kinases, including ABL1 and ABL2, transduce diverse extracellular signals to protein networks. Recently, Morita et al. showed that the non-receptor ABL tyrosine kinases enhanced the enzymatic activities of the endoplasmic reticulum (ER) transmembrane kinase, IRE1a, thereby potentiating ER stress-induced apoptosis.
ER stress has been implicated in the pathogenesis of idiopathic pulmonary fibrosis (IPF). IPF is a disease of progressive fibrosis and respiratory failure. ER stress activates a signaling pathway called the unfolded proteins response (UPR). UPR either restores homeostasis or promotes apoptosis. Cells operate a signaling network termed unfolded protein response (UPR) to monitor protein-folding capacity in the ER.
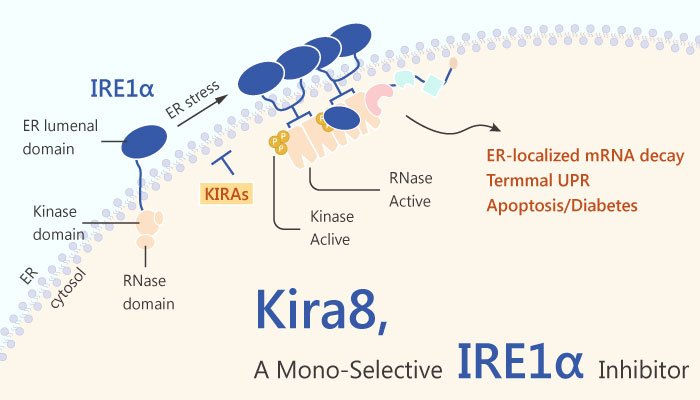
Inositol-requiring enzyme 1 (IRE1) is an ER transmembrane sensor that activates the UPR to maintain the ER and cellular function. Although mammalian IRE1 promotes cell survival, it can initiate apoptosis via the decay of antiapoptotic miRNAs. Furthermore, convergent and divergent IRE1 characteristics between plants and animals underscore its significance in cellular homeostasis. Small-molecule IRE1α kinase-inhibiting RNase attenuators (KIRAs) evaluates the importance of IRE1α activation in Bleomycin-induced mouse pulmonary fibrosis.
Hopefully, Mono-selective IRE1α inhibitor-Kira8 attenuates IRE1α pharmacologically in the Akita mouse preserves glycemic control in this monogenetic, fulminant diabetes model. Besides, Kira8 more potently reduces IRE1α-driven apoptosis in INS-1 cells than Kira6. Kira8 also reverses XBP1 splicing promoted by GNF-2. The X-box binding protein 1 (XBP1) is a transcription factor containing a bZIP domain. Kira8 blocks IRE1α oligomerization, and potently inhibits IRE1α RNase activity against XBP1 and Ins2 RNAs.