Protease-activated receptors (PARs) are a family of four G-protein-coupled receptors for proteases from the circulation, inflammatory cells and epithelial tissues. Of these, PAR2 is one of four known proteinase activated receptors. Crucially, PAR2 plays an important role in inflammation and pain. Besides, PAR2 also is important in inflammation and proliferation. PAR2 ligands can differentially regulate activation, desensitization and internalization, and such selective ligands could be valuable probes for dissecting signalling pathways associated with different specific diseased states. Especially, proteases cleave PAR2 to expose a tethered ligand that binds to the cleaved receptor. Moreover, activation of PAR2 mediates inflammation in the peripheral nervous system, which results in neurogenic inflammation and hyperalgesia. A range of serine proteases, such as trypsin, specifically activate PAR2.
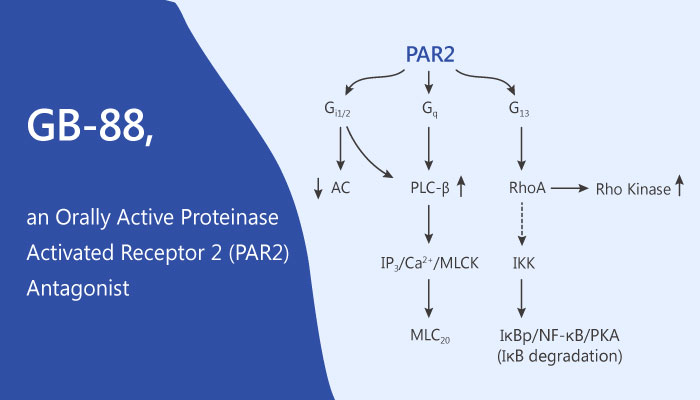
In this study, JY Suen, et al reported some properties of a novel PAR2 antagonist GB-88. Firstly, GB-88 is a unique PAR2 antagonist, which inhibits PAR2 activated Ca2+ release (IC50∼2 µM) induced by native (trypsin) or synthetic peptide and non-peptide agonists. GB-88 also is an orally active inhibitor of PAR2 activation in vivo with anti-inflammatory activity in the rat. GB-88 inhibits PAR2-induced acute inflammation in vivo. In particular, GB-88 is orally active and anti-inflammatory in vivo. As a result, GB-88 inhibits acute rat paw oedema elicited by agonist GB110 and proteolytic or peptide agonists of PAR2 but not by corresponding agonists of PAR1 or PAR4. All in all, GB-88 regulates PAR2 activity in vitro and in vivo.