Innate immune sensing in the tumor microenvironment (TME) is a critical step in promoting spontaneous tumor-initiated T cell priming and subsequent tumor-infiltrating lymphocytes (TILs) infiltration. Spontaneous tumor-initiated T cell priming is dependent on IFN-β production by tumor-resident dendritic cells. In particular, activating STING induces the signature cytokine IFN-β. Activation of the STING pathway in tumor-resident host antigen presenting cells (APCs) is required for induction of a spontaneous CD8+ T cell response against tumor-derived antigens in vivo. Bacterial infection produces exogenous cyclic dinucleotides (CDNs), and host cyclic GMP-AMP synthetase (cGAS) produces a structurally distinct endogenous cyclic dinucleotide. In this study, Leticia Corrales, et al selected ADU-S100 (ML RR-S2 CDA) as the lead molecule for continued development. Especially, ADU-S100 is a strong agonist of the mSTING pathway in vitro and in vivo.
ADU-S100 has high translational potential as a therapeutic intervention strategy to induce activation of the tumor microenvironment in multiple tumor types. Particularly, ADU-S100 has the mechanistic goal of generating effective tumor-initiated CD8+ T cell priming and lasting antitumor efficacy. ADU-S100 also induces aggregation of STING and induce phosphorylation of TBK1 and IRF3 in mouse bone marrow-derived macrophages (BMM). Besides, ADU-S100 induces significantly high levels of IFN-α. Importantly, ADU-S100 also shows high anti-tumor control than the endogenous ML cGAMP. ML RR-S2 CDA induces lasting immune-mediated tumor rejection in multiple tumor types. Together, ADU-S100 promotes immune-mediated tumor rejection.
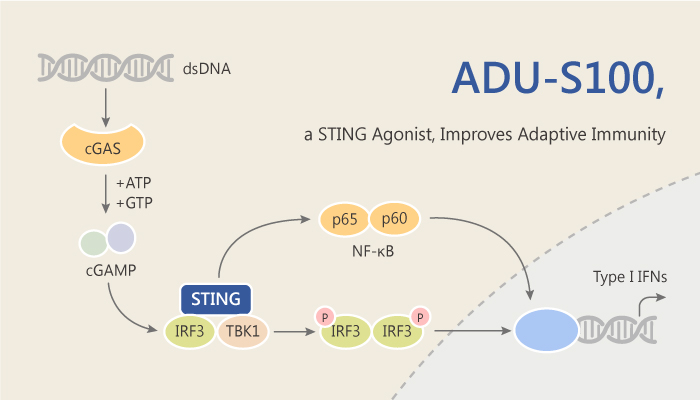
All in all, these results demonstrate that ADU-S100 eradicates multiple tumor types and primes an effective systemic CD8+ T cell immune response that significantly inhibits the growth of distal, untreated lesions.