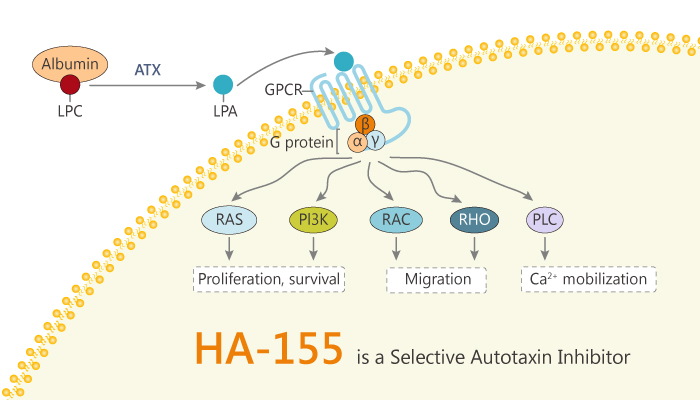
Autotaxin (ATX) is a secreted lysophospholipase D which generates the bioactive lipid mediator lysophosphatidic acid (LPA). It is responsible for the hydrolysis of lysophosphatidylcholine (LPC) into lysophosphatidic acid (LPA) and choline. It has been reported that LPA stimulates migration, proliferation, and survival of cells by activating specific G protein-coupled receptors. Additionally, the ATX-LPA signaling axis is involved in cancer, inflammation, and fibrotic disease.
ATX binds to integrins, Whatmore, ATX plays a role for the solvent-exposed surface of the N-terminal tandem somatomedin B-like domains in binding to platelet integrin αIIbβ3.
The opposite face of the somatomedin B-like domain interacts with the catalytic phosphodiesterase (PDE) domain to form a hydrophobic channel. Lysophospholipid substrates can enter and leave the active site through the channel. Therefore, potent and selective ATX inhibitors are necessary to elucidate the contribution of ATX action to signaling cascades.
In this article, we will introduce a selective boronic acid-based ATX inhibitor, HA-155.
This compound possesses a boronic acid moiety in the inhibitor structure to rationally target the threonine oxygen nucleophile of ATX with a hard matching Lewis acid.
Firstly, Its crystal structure in complex ATX shows that HA-155 targets the threonine oxygen nucleophile in the ATX active site via the boronic acid moiety. Meanwhile, the hydrophobic 4-fluorobenzyl moiety of HA-155 targets the hydrophobic pocket responsible for lipid binding.
Nextly, platelets are important participants in LPA production in the circulation. LPA levels in serum from platelet-rich plasma are ∼5-10-fold higher than in platelet-poor plasma. Therefore, activated-platelets play an active role in LPA production during clotting. At the same time, an anti-platelet antibody can decrease the production of LPA in serum by almost 50% in rat experiments.
Lastly, several observations suggest that platelets can serve as a useful model system to research the regulation of ATX activity. Isolated platelets produce LPA after agonist stimulation in a process that presumably serves as a substrate for ATX.
There exists a data about the effects of the small molecular inhibitor HA-155 of ATX. An effective dose of thrombin (0.5 units/ml) increased the LPA content of isolated platelets ∼6-fold after 30 min. Subsequently, the ATX inhibitor HA155 completely attenuates the thrombin-mediated increase in platelet-derived LPA as a dose-dependent manner.
In summary, HA155, as a novel Boronic acid-based inhibitor, which inhibits autotaxin by binding to the ATX active site.
Reference:
Albers HM, et al. Chem Rev. 2012 May 9;112(5):2593-603.
Fulkerson Z, et al. J Biol Chem. 2011 Oct 7;286(40):34654-63.