Metastasis is the major driver in cancer-related deaths. Metastases involve dysregulation of numerous cellular processes. Importantly, blockade of RhoC prevents cellular invasion in breast and prostate cancer models. Especially, RhoA is a small GTPase protein in the Rho family of GTPases. RhoA is a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression, and cell transformation. Furthermore, a knockout mouse shows complete suppression of in vivo metastasis of polyoma T-antigen-induced mammary tumors. As a result, the role of Rho-regulated gene transcription by serum response factor (SRF) and its transcriptional cofactor myocardin-related transcription factor A (MRTF-A) suggest a locus for pharmacological intervention. In this study, researchers reported the design and synthesis of CCG-203971.
Transforming protein RhoA, also known as Ras homolog family member A (RhoA)/Rho-associated coiled-coil forming protein kinase signaling is a key pathway in multiple types of solid organ fibrosis. CCG-1423 is the first-generation inhibitor of Rho/MKL1/SRF-mediated gene transcription that inhibits invasion of PC-3 prostate cancer cells in a matrigel model of metastasis. CCG-203971 is a second-generation RhoA/MRTF/SRF inhibitor with improved selectivity for inhibiting invasion vs acute cytotoxicity. In particular, CCG-203971 inhibits PC-3 cell migration with an IC50 of 4.2 μM.
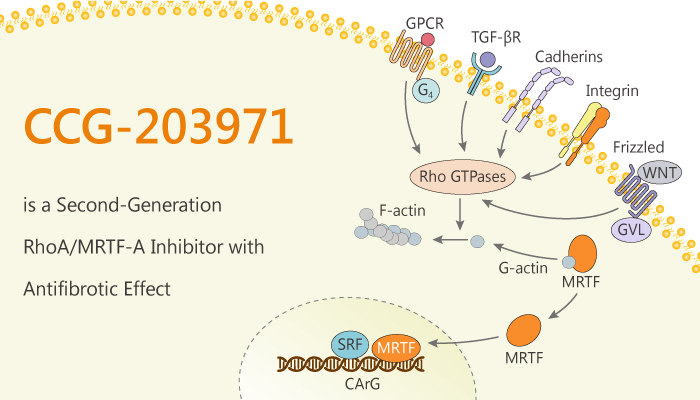
CCG-203971 inhibits pro-fibrotic signaling and a large body of literature exists that suggests this is through MRTF/SRF. TGF-β signaling activates RhoA and MRTF/SRF gene transcription through a non-canonical pathway. Moreover, CCG-203971 significantly represses TGF-β– induced MKL1 expression at 17.5 and 25 μM concentrations. Besides, CCG-203971 represses MYLK mRNA levels in a concentration-dependent manner.
All in all, CCG-203971 is an inhibitor of the RhoA/MRTF/SRF transcriptional pathway as a potential anti-metastasis agent.