Tubulointerstitial fibrosis is a chronic and progressive process affecting kidneys during aging and in chronic kidney disease (CKD). The US Centers for Disease Control and Prevention project that 47% of 30-year-olds will develop CKD during their lifetime. Moreover, 11% of individuals with stage 3 CKD will eventually progress to end-stage renal disease (ESRD), requiring dialysis or kidney transplantation.
CKD is one of the strongest risk factors for cardiovascular disease. However, available treatments to slow CKD progression and prevent CKD-related complications are quite limited and include angiotensin-converting enzyme inhibition, angiotensin receptor blockade, optimal blood pressure control, and sodium bicarbonate for metabolic acidosis. Despite these therapies, outcomes in CKD remain poor. Hence, we will introduce a phosphodiesterase (PDE) inhibitor-IBMX.
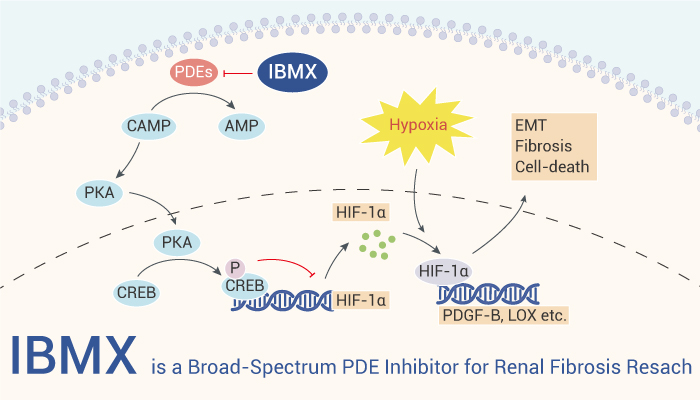
IBMX is a broad spectrum and non-selective PDE inhibitor.
IBMX shows IC50 values of 6.5, 26.3 and 31.7 µM for PDE3, 4 and 5, respectively.
IBMX (200, 400, 800 μM; 2, 6, 24 h) reduces HIF-1α mRNA in human proximal tubular epithelial HK-2 cells. In addition, IBMX (400 μM; 30 min) reduces HIF-1α mRNA through cAMP–PKA mediated pathway. Besides, IBMX (200, 400, 800 μM; 2, 6, 24 h) downregulates hypoxia induced markers of renal fibrosis in HK-2 cells. Also, IBMX (400 μM; 4, 24, 48 h) attenuates hypoxia induced cell-death and EMT in HK-2 cells. Taken together, the PDE inhibitor IBMX can downregulate the transcription of HIF-1α, and thus may attenuate hypoxia induced renal fibrosis.
IBMX not only shows good activity in renal fibrosis, but also in anti-cancer.
Glioma stem cells (GSCs), a small subpopulation of cells with stemlike properties, are the real driving force for tumor initiation, progression, and relapse. When IBMX in combination with forskolin (Fsk), upregulats the expression of cAMP related protein pCREB in GSCs. In addition, Fsk‐IBMX inhibit proliferation and promoted apoptosis of GSCs.
In a word, IBMX is a promising PDE inhibitor for renal fibrosis and cancer research.
Reference:
[1] Hasan AU, et al. Exp Cell Res. 2017 Sep 15;358(2):343-351.
[2] Lv P, et al. J Cell Biochem. 2019 Jan;120(1):321-331.
[3] Benjamin D. Humphreys. Annu Rev Physiol. 2018 Feb;80:309-326.