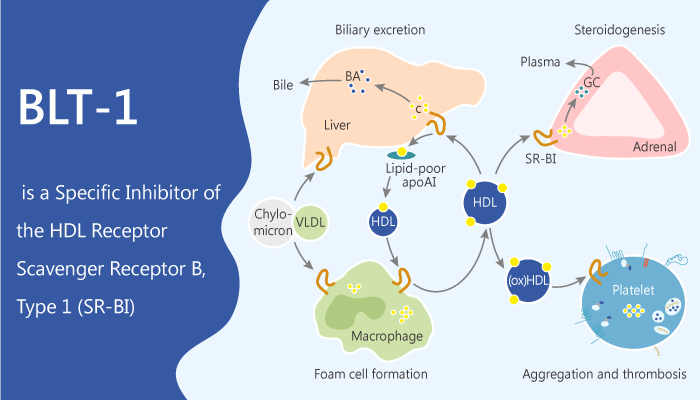
SR-BI is the HDL1 receptor and plays a key role in lipoprotein metabolism. In mice, SR-BI distributes in hepatocytes, it controls plasma lipoprotein metabolism. Besides, in steroidogenic cells, SR-BI enables the delivery of cholesterol for storage and steroidogenesis. Therefore, SR-BI expression protects against atherosclerosis and it plays an important role in lipoprotein metabolism.
SR-BI affects the binding of HDL to cells and its transfer into the cells of cholesteryl esters from HDL’s core. This is a selective lipid uptake process, it is found on the cell surface. Also, this process is different from the delivery of lipoprotein cholesterol to cells via the LDL receptor.
In this article, we will introduce a small-molecule inhibitor of SR-BI-dependent selective lipid uptake, BLT-1. BLT-1 blocks lipid transport and inhibits selective uptake from HDL of either cholesteryl ethers.
Firstly, in an uptake assay, BLT-1 inhibits SR-BI-mediated lipid transport. And the IC50s for cellular DiI-HDL and CE-HDL uptake in ldlA[mSR-BI] and ldlA-7 cells are 21 nM and 57 nM, respectively.
Secondly, BLT-1 can inhibit SR-BI-dependent selective uptake of [3H]CE from [3H]CE-HDL by mSR-BI-t1-containing liposomes in cells (IC50=0.057 µM) and liposomes (IC50=0.098 µM)
BLT-1 inhibits SR-BI-mediated lipid transport, at the same time, it increases SR-BI’s binding affinity for HDL.
BLT-1 inhibits the transfer of lipids between HDL and cells mediated by the HDL receptor SR-BI. It inhibits cellular selective lipid uptake of HDL cholesteryl ether. At the same time, it has an inhibitory effect on the efflux of cellular cholesterol to HDL. BLT-1 possesses high specificity, it needs SR-BI but has nearly no affects on the secretory pathway, or the actin or tubulin cytoskeletal networks. Notably, the inhibitory effects of BLT-1 accompany with high affinity to HDL.
BLT-1, a thiosemicarbazone copper chelator, is a selective scavenger receptor B, type 1 (SR-BI) inhibitor. BLT-1 inhibits the transfer of lipids between high-density lipoproteins (HDL) and cells mediated by SR-BI.
In conclusion, BLT-1, as a thiosemicarbazone copper chelator, is a highly selective SR-BI inhibitor. Given the strong inverse correlation between the effects of BLTs on binding and lipid transport. This agent has the potential for the investigation of disrupted lipid metabolism.
Reference:
Nieland TJ, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15422-7.