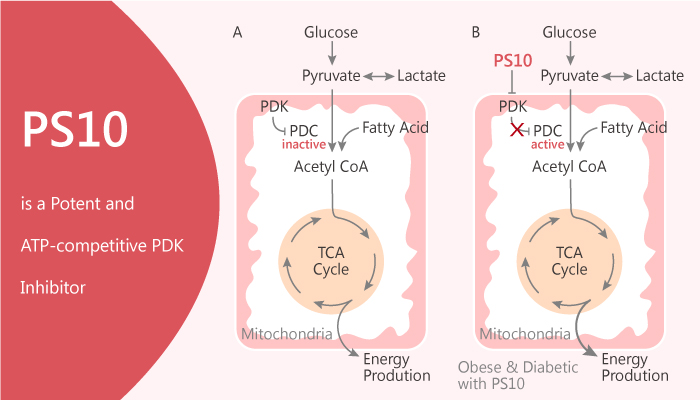
The mitochondrial pyruvate dehydrogenase complex (PDC) is a protein complex, contains E2 and the E3-binding protein (E3BP). Besides, the isoforms of pyruvate dehydrogenase kinase (PDKs 1–4) and pyruvate dehydrogenase phosphatase (PDPs 1–2) interacts with each other through ionic attachments. The PDC contributes to the production of acetyl-CoA, then participants in the Krebs cycle.
PDC plays an important role in the regulation of PDC activity. Meanwhile, it is also critical for glucose homeostasis and fuel selection in the glucose-fatty acid cycle. The phosphorylation of PDC leads to inactivation, while PDP restores PDC activity and induces dephosphorylation of PDC activity.
The PDKs overexpression always increases in many disease states such as diabetes, cancer, and heart failure.
In this article, we will introduce a robust PDK inhibitor, PS10.
Firstly, among the ATP-competitive PDK inhibitors, the Kd value of 239 nm for PS10 binding to PDK2 is the lowest. Also, PS10 is less potent than cycloheximide in HeLa cells. It shows an IC50 value of 284 μM for growth inhibition.
Nextly, in a PK study, after a single dose injection of PS10, the terminal t½, Cmax, Tmax, AUC last, and Vz/F values are 161 min, 32,400 ng/ml are 1,905, 10 mins, 136 min ng/ml and 172 ml, respectively.
In DIO mice model, PS10 leads to 11- and 23-fold higher PDC activity in the heart and liver, respectively. Meanwhile, there results in a 1.4-fold enhancement of PDC activity in kidneys compared with the vehicle-group.
Lastly, PS10 can increase glucose tolerance in DIO mice. When the control-mice at the treatment of 1.5 g/kg glucose, the plasma glucose level in mice is at 200 mg/dl at 0 min, peaks at 482 mg/dl at 30 min, and reduces to 210 mg/dl at 120 min. In PS10-treated DIO mice, the glucose level at 168 mg/dl at 0 min is lower than that in vehicle-treated animals. Besides, it reaches 312 mg/dl at 30 min and returns to 163 mg/dl at 120 min.
In conclusion, PS10 is a novel, potent and ATP-competitive pan-PDK inhibitor, inhibits all PDK isoforms in vitro. And it can improve glucose tolerance and correct metabolic dysfunction in vivo.
Reference:
Tso SC, et al. J Biol Chem. 2014 Feb 14;289(7):4432-43.