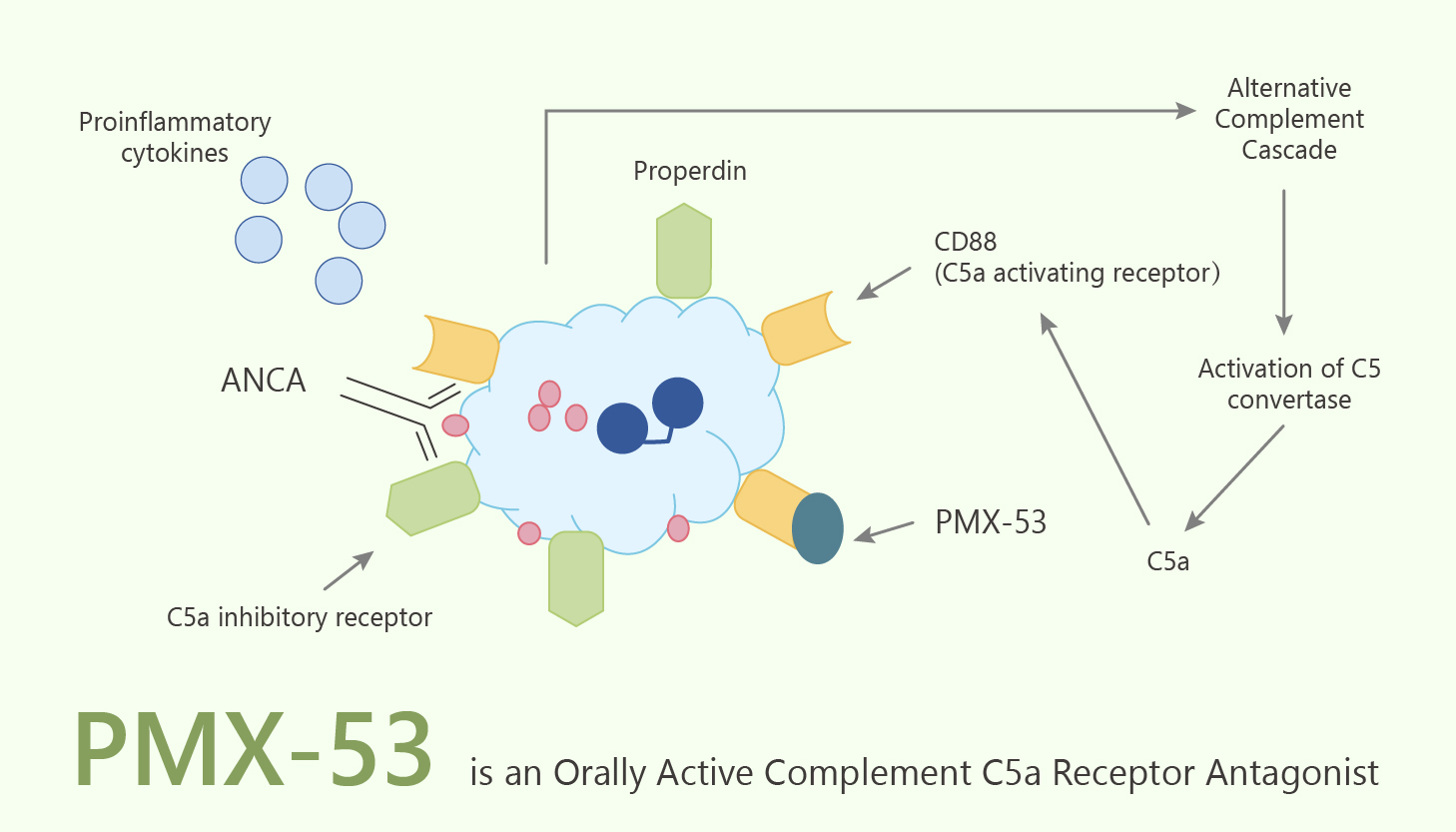
C5a is an important inflammatory hypernociceptive mediator, acting by a mechanism independent of hypernociceptive cytokine release, but dependent on the presence of neutrophils. Especially, C5a is a complement activation product. Moreover, C5a exhibits a broad spectrum of inflammatory activities. C5a receptor modulates inflammatory responses, obesity, development and cancers. The anaphylatoxin C5a interacts with its cognate cell surface G protein-coupled receptor (GPCR; CD88) to activate neutrophils and macrophages. In particular, PMX-53 is a potent CD88 antagonist. PMX-53 inhibits C5a-induced neutrophil myeloperoxidase release and chemotaxis with IC50 values of 22 and 75 nM, respectively. In addition, PMX-53 functions as a potent CD88 antagonist and a low-affinity agonist for MrgX2.
PMX-53 (10 nM) inhibits C5a-induced Ca2+ mobilization in HMC-1 cells, but at higher concentrations (≥30 nM) it causes degranulation in LAD2 mast cells, CD34+ cell-derived mast cells, and RBL-2H3 cells stably expressing MrgX2. Consistent with degranulation, PMX-53 induces Ca2+ mobilization at concentrations of 100 nM. PMX-53 is a dual-action molecule; it is a high-affinity antagonist of CD88 but a low-affinity agonist of MrgX2.
In vivo, local pretreatment of rats with PMX-53 (60-180 μg per paw) inhibits zymosan-, carrageenan-, lipopolysaccharide (LPS)- and antigen-induced hypernociception.
Furthermore, PMX-53 displays a potent peripheral anti-hypernociceptive effect in different inflammatory models, both in rats and mice. Treating animals with PMX-53 addresses the importance of C5a in the genesis of inflammatory hypernociception.
To summarise, PMX-53 is a selective C5a receptor antagonist. Inhibiting the action of C5a has therapeutic potential in the control of inflammatory pain. As a resultPMX-53 is a potent CD88 antagonist in human neutrophils and macrophages in vitro and protects rodents from a number of experimental inflammatory diseases.