iKIX1 is an anti-fungal agent and re-sensitizes drug-resistant C. glabrata to azole antifungals in vitro.
In HepG2 cells, iKIX1 inhibits cell growth in a concentration-dependent manner in the presence of 5 µM Ketoconazole (KET). FP titration curve showing the interaction of the CgGal11A KIX domain with CgPdr1 AD30 fitted to a Kd of 319.7 nM. iKIX1 competes with CgPdr1 AD30 with an IC50 of 190.2 µM. Additionally, In vitro binding studies, iKIX1 reveals that the Kd of the CgPdr1 activation domain (AD) for the CgGal11A KIX domain is 0.32 µM and the apparent Ki for iKIX1 is 18 µM.
iKIX1 inhibits ketoconazole (KET)-induced upregulation of luciferase activity in a dose-responsive manner in an Sc pdr1Δpdr3Δ strain containing plasmid-borne CgPDR1 and 3XPDRE-luciferase.
A chromatin immunoprecipitation (ChIP) assay is used to examine Gal11/Med15 recruitment to Pdr1-regulated target genes in S. cerevisiae. Ketoconazole induces Gal11/Med15 rapidly recruited to the promoters of the Pdr1 target genes PDR5 and SNQ2.
However, iKIX1 abrogates Ketoconazole-induced recruitment of Gal11/Med15 and strongly inhibits azole-induced transcription of ScPdr1 target genes. T iKIX1 has an effect on the transcription of C. glabrata Pdr1-regulated genes involved in drug efflux and MDR (CgCDR1, CgCDR2, and CgYOR1). This compound alone does not significantly affect Pdr1-target gene induction. But pre-treatment with iKIX1 reduces ketoconazole-induced CgPdr1 up-regulation in a durable and concentration-dependent manner.
In RNA sequencing (RNA-Seq) assay of a C.glabrata SFY114 (PDR1 wild-type) strain. Azole up-regulates Pdr1-dependent genes in both yeasts, such as the drug efflux pumps ScPDR5 and CgCDR1i.
iKIX1 combines azole strongly blunts expression of many azole-activated and Pdr1-dependent genes in both S. cerevisiae and C. glabrata. But iKIX1 alone affects very different sets of genes in S. cerevisiae and C. glabrata. And then iKIX1 does not significantly alter the expression of PDR1 or GAL11/MED15 affects very different sets of genes in S. cerevisiae and C. glabrata.
iKIX1 restores the efficacy of azoles towards CgPDR1 gain-of-function mutants. It restores azole-sensitivity to PDR1 gain-of-function mutant strains in a concentration-dependent manner.
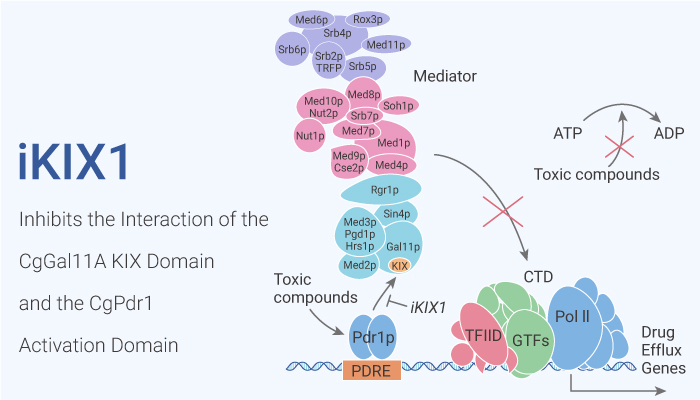
In conclusion, iKIX1 is an inhibitor of the interaction between the KIX domain of the mediator subunit CgGal11A and the activation domain of CgPdr1. It is used for the study of multidrug resistance and C. glabrata infection.
Reference:
Joy L Nishikawa, et al. Nature. 2016 Feb 25;530(7591):485-9.