Members of the activating protein 1 (AP-1) and nuclear factor-κB (NF-κB) families of transcription factors plays an important role in many essential physiological and pathological processes. These transcription factors are key regulators of the inducible expression of many pro-inflammatory mediators.
JNKs directly involves controlling the regulation of AP-1 transcriptional activity. Therefore, the development of JNK inhibitors are focused on the research of chronic inflammatory diseases
One of the targets of activated JNKs is c-Jun. When c-Jun phosphorylates on Ser63 and/or Ser73, this protein is capable of binding AP-1 sites in the nucleus. JNKs also phosphorylate other AP-1 proteins, including JunB, Jun D, and ATF2. Although AP-1 and NF-κB are involved in different signaling pathways. However, the cross-talk between these pathways occurs in part by the ability of certain Jun and Fos family proteins to interact with the p65 subunit of NF-κB.
In this article, we will introduce a specific inhibitor of the c-Jun N-terminal kinase (JNK) family, IQ-3.
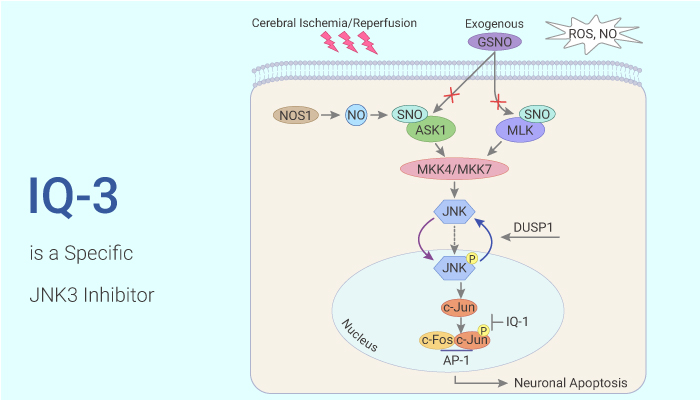
IQ-3 is an inhibitor of the JNK family with a preference for JNK3. IQ-3 exhibits Kd values of 0.24 μM, 0.29 μM, and 0.066 μM for JNK1, JNK2, and JNK3, respectively.
In different cells, IQ-3 exhibits IC50 of 2.2 μM (TNF-α in human mono mac-6 cells), 1.5 μM (IL-6 in human mono Mac-6 cells), 4.7 μM (TNF-α in human PBMCs), respectively. Additionally, It also shows IC50 values of 9.1 μM (IL-6 in human PBMCs) and 6.1 μM (NO in murine J774.A1), respectively.
In human THP-1 Blue monocytic cells, IQ-3 exhibits an IC50 of 1.4 μM for inhibiting LPS-induced NF-κB/AP-1 transcriptional activity.
JNKs and CK1δ are the main kinase targets of IQ-3, but the nanomolar binding affinity for JNK3 is approximately 10-fold higher than for CK1δ. Additionally, IQ-3 demonstrates favorable pharmacokinetics and inhibits ovalbumin-induced CD4+ T-cell immune responses in vivo.
In conclusion, IQ-3 is indeed a competitive inhibitor for the ATP binding site of JNK3.
Reference:
Igor A Schepetkin, et al. Mol Pharmacol. 2012 Jun;81(6):832-45.