The U.S. Food and Drug Administration approved Zuranolone, the first oral medication indicated to treat postpartum depression (PPD) in adults.
PPD is a major depressive episode that typically occurs after childbirth but can also begin during the later stages of pregnancy. Until now, treatment for PPD was only available as an IV injection given by a health care provider in certain health care facilities. As with other forms of depression, PPD can present with symptoms such as cognitive impairment, feelings of sadness or inadequacy, loss of energy or suicidal ideation.
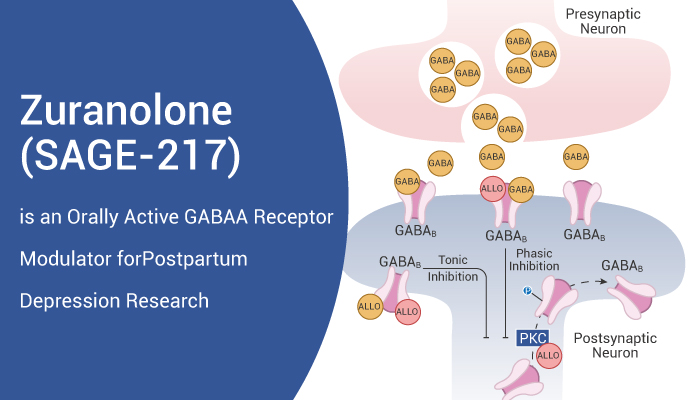
Zuranolone is a potent GABAA receptor agonist
Zuranolone is an orally active and potent neuroactive steroid positive allosteric modulator of GABAA receptor, with EC50s of 296 and 163 nM for α1β2γ2 and α4β3δ GABAA receptors, respectively.
In parallel phase 2 clinical trials for the treatment of postpartum depression (PPD) and major depressive disorder (MDD), Firstly, Zuranolone shows >30 μM inhibition in a cardiac panel of eight relevant cardiac ion channels. Secondly, at 10 μM concentration of Zuranolone, only binding at the glycine (57%), sigma receptors (88%), and inhibition of the transient receptor potential vanilloid 1 (TRPV1, 95%) is noted. Meanwhile, acute administration of Zuranolone (0.3 to 10 mg/kg, i.p.) effectively reduces pentylenetretazol (PTZ)-induced seizures in mice (MECplasma=85 nM) as well as produces a dose-dependent anticonvulsant effect in the mouse 6 Hz electrical stimulation model.
Furthermore, in the rat model of status epilepticus (SE), Zuranolone (0.3 to 5 mg, i.v.) abolishes both behavioral and electrographic seizure activity, even when administered 60 min after induction of SE. Finally, additional PK studies of Zuranolone in dog show low clearance (<10% of hepatic blood flow), resulting in excellent oral bioavailability (F=68%).
Reference:
[1] Martinez Botella G, et al. J Med Chem. 2017 Sep 28;60(18):7810-7819.