Parkinson’s disease (PD) is the second common progressive neurodegenerative disorder next to Alzheimer’s disease. The pathological hallmark of Parkinson’s disease includes progressive loss of dopaminergic cells present in the substantia nigra pars compacta in brain.
Monoamine oxidase B (MAOB) is expressed in the mitochondrial membrane and has a key role in degrading various neurologically active amines such as benzylamine, phenethylamine and dopamine with the help of Flavin adenine dinucleotide (FAD) cofactor. MAOB is a well-established therapeutic target for PD. The Parkinson’s disease associated symptoms can be treated using inhibitors of MAOB as the dopamine degradation can be reduced. Here, we will introduce a reversible MAOB inhibitor — Tisolagiline.
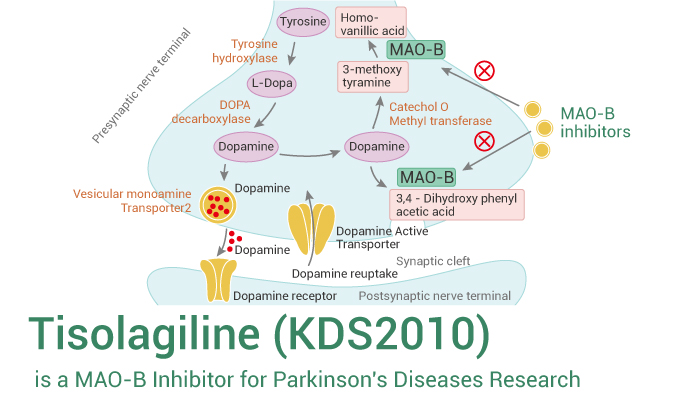
Tisolagiline (KDS2010) is a reversible MAOB inhibitor for Parkinson’s disease research.
In a research, scientists tested the therapeutic potential of KDS2010, a recently synthesized potent, selective, and reversible MAOB inhibitor in multiple animal models of PD. They designed and synthesized a series of o-aminoamide derivatives and found that derivative KDS2010 exhibited the highest potency, specificity, reversibility, and bioavailability (> 100%). In addition, KDS2010 demonstrated significant neuroprotective and anti-neuroinflammatory efficacyagainst nigrostriatal pathway destruction in the mouse MPTP model of parkinsonism. Treatment with KDS2010 also alleviated parkinsonian motor dysfunction in 6-hydroxydopamine-induced and A53T mutant a-synuclein overexpression rat models of PD. Moreover, KDS2010 showed virtually no toxicity or side effects in non-human primates. KDS2010 could be a next-generation therapeutic candidate for PD.
Reference:
[1] Nam MH, et al. Neurotherapeutics. 2021 Jul;18(3):1729-1747.
[2] Murugan NA, et al. Int J Mol Sci. 2020 Oct 16;21(20):7648.