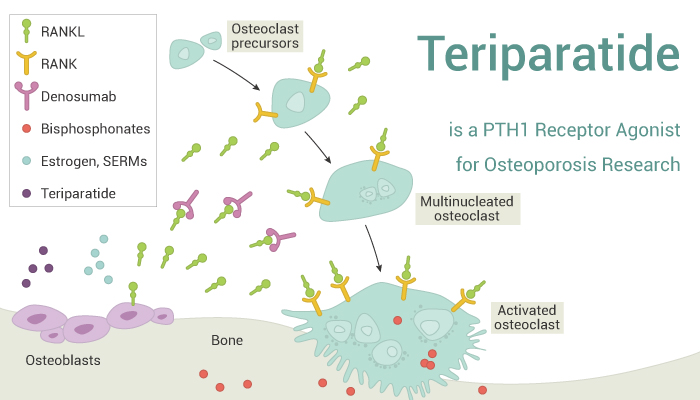
Osteoporosis is a systemic skeletal disease. Characterised by reduced bone mineral density (BMD) and weakened bone structure, osteoporosis decreases bone resistance to low-energy trauma and increases bone fragility and fracture risk. Almost all pharmacological agents for osteoporosis specifically target the bone resorption component of bone remodelling pathways. Therefore, they are classified as anticatabolic or antiresorptive agents (e.g. denosumab). The only anabolic agent currently available is teriparatide (Z). These treatments reduce the risk of osteoporotic fractures and stabilise or increase bone mass and strength.
Teriparatide is a PTH1 receptor agonist for osteoporosis research.
There are two main pharmacological approaches to osteoporosis: (1) anabolic therapy, which stimulates new bone formation; (2) anticatabolic or antiresorptive therapy, which decreases bone resorption and/or inhibits bone turnover.
On the one hand, Teriparatide, a recombinant fragment of parathyroid hormone, stimulates bone formation by increasing osteoblast activity. To a lesser extent, it inhibits osteoclast recruitment. Teriparatide is the sole anabolic agent approved for treating postmenopausal osteoporosis.
On the other hand, antiresorptive therapy reduces bone turnover by targeting osteoclasts. Firstly, estrogen replacement therapy and likewise the SERM raloxifene interfere with various osteoblast-derived factors that stimulate osteoclasts (e.g. IGF1, TGF-β and TNF-α). Secondly, Denosumab binds the cytokine RANKL, preventing it from binding its receptor, RANK. Besides, Denosumab prevents maturation of osteoclast precursors and promotes apoptosis of mature, multinucleated osteoclasts. Furthermore, bisphosphonates bind to bone mineral and are taken up by osteoclasts, causing them to undergo apoptosis or have reduced resorptive capacity. When osteoclast number and activity decline, bone formation eventually slows to maintain a balance of bone resorption and formation.
Reference:
[1] Hanley DA, et al. Int J Clin Pract. 2012 Dec;66(12):1139-46.
[2] Iwamoto J, et al. Calcif Tissue Int. 2016 Nov;99(5):535-542.