The blockade of the PD-1/PD-L1 immune checkpoint pathway with monoclonal antibodies has provided significant advances in cancer therapy. However, the antibody-based immunotherapies carry a number of disadvantages, including the high cost of the antibodies, their short half-life, and immunogenicity. The development of small-molecule PD-1/PD-L1 inhibitors could overcome these drawbacks. But the related studies were lacked due to the incomplete structural information for this pathway. A study from Katarzyna Guzik firstly reported a potent PD-1/PD-L1 interaction inhibitor, BMS-1166.
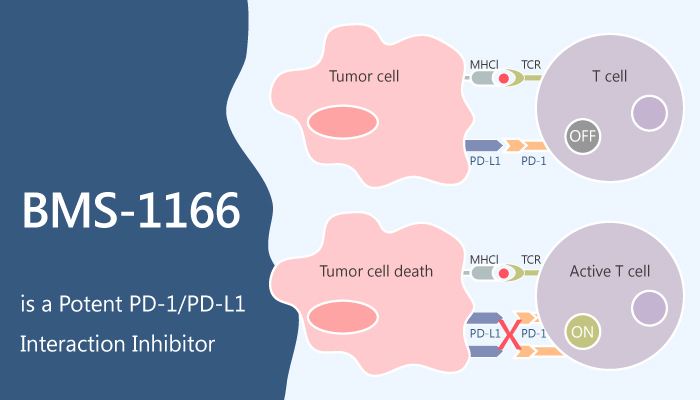
In vitro, BMS-1166 represents a potent PD-1/PD-L1 interaction inhibitor with IC50 of 1.4 nM in a homogenous time-resolved fluorescence binding assay. Additionally, BMS-1166 antagonizes the inhibitory effect of PD-1/PD-L1 immune checkpoint on T cell activation. Besides, BMS-1166 dose-dependently abolishes the inhibition of ECs stimulation by sPD-L1.
Another study from Lukasz Skalniak suggested that the tested BMS compounds force the conformational changes at the surface of the PD-L1 molecule upon binding. Both conformational changes and additional interaction sub-sites absent in previously published structures result in improved interaction between the characterized compounds and the target protein. These results prove that although the targeting of PD-1/PD-L1 interaction with small molecules was considered challenging due to the relatively flat interface of PD-1/PD-L1 interaction, it is not unfeasible.
Collectively, scientists advocate for PD-1/PD-L1-blocking potential of the evaluated BMS compounds and present a likely mode of this inhibition by forcing the dimerization of PD-L1 molecules.