PI3Ks are key drivers of the survival and growth of normal B cells. PI3K catalyzes the phosphorylation of PI(4,5)P2 to generate PI(3,4,5)P3. It serves as a docking site at the plasma membrane for various pleckstrin homology domain-containing proteins, including phospholipase Cγ2 (PLCγ2), BTK, and AKT. These effectors can activate and drive downstream signaling leading to cell growth and survival. PI3K activity is countered by the phosphatases PTEN and SHIP1 and 2. In addition to their catalytic functions, SHIP1/2 may also modulate signaling by acting as scaffold proteins. Multiple signaling pathways converge on PI3Ks in various subtypes of B-cell neoplasms, including signaling downstream of the B-cell receptor (BCR), a major driver, and therapeutic target for malignant B-cells. In this study, AQX-435 is a novel small molecule SHIP1 agonist. It reduces PI3K activation downstream of the B-cell receptor (BCR) and induces apoptosis of malignant B cells, and reduces lymphoma growth.
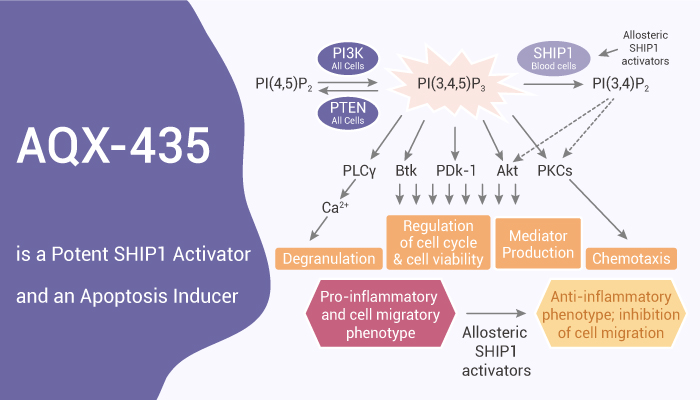
AQX-435 reduces PI3K activation downstream of the BCR and induces apoptosis of malignant B cells and reduces lymphoma growth.
AQX-435 reduces CLL cell viability in a dose-dependent manner. AQX-435-induced apoptosis was mediated via caspases since AQX435 induced PARP cleavage. It effectively inhibits PI(3,4,5)P3-mediated signaling downstream of the BCR in CLL and DLBCL cells. AQX-435 and Ibrutinib combine effectively to enhanced inhibition of BCR signaling. It also induced TMD8 cell apoptosis in vitro with an IC50 of ~2 µM. Moreover, AQX-435 reduces anti-IgM-induced AKT phosphorylation and induces apoptosis in DLBCL cells. Furthermore, it significantly reduced the volume of TMD8 tumors. In addition, AQX-435 inhibits DLBCL PDX tumors growth. It also reduced AKT phosphorylation and growth of DLBCL in vivo and cooperated with ibrutinib for tumor growth inhibition.
In summary, AQX-435 also co-operated with the BTK inhibitor Ibrutinib to enhance inhibition of anti-IgM-induced AKT phosphorylation. AQX-435 induces caspase-dependent apoptosis of CLL cells preferentially as compared to normal B cells and overcame in vitro survival promoting effects of microenvironmental stimuli. SHIP1 activation may be an effective novel therapeutic strategy for the treatment of B-cell neoplasms.
Reference:
Lemm EA, et al. Clin Cancer Res. 2020;26(7):1700-1711.