Receptor interacting protein kinase-1 and -3 (RIP1 and RIP3) are serine/threonine protein kinases. In addition, RIP1 and RIP3 share a conserved kinase domain. The protein-protein interaction between RIP1 and RIP3 is an essential signaling step to initiate necroptosis in most cell types. The two kinases are essential mediators of cell death processes and participate in inflammatory responses. RIP1 binds to death receptors including tumor-necrosis factor receptor 1. RIP1 also triggers the formation of various signaling complexes. These complexes promote cell survival or apoptosis depending on the cell type and cellular contents. In various cell types, RIP1 and RIP3 regulate expression of pro-inflammatory cytokines. The regulation mechanisms are independent of necrosis and the subsequent release of danger-associated molecular pattern molecules.
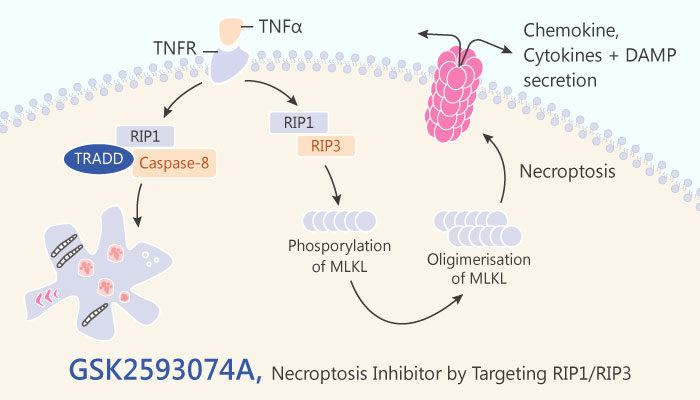
Necrostatin-1 is the first proven RIP1 inhibitor. Necrostatin-1 probes RIP1 functions in pathogenesis of multiple human disease models. In this study, Ting Zhou, et al screened 1141 kinase inhibitors for their ability to block necroptosis, using Necrostatin-1 as a selection baseline. Positive compounds were further screened for cytotoxicity and virtual binding to RIP3. Among them, GSK2593074A completely blocks necroptosis in both human and murine cells at 10 nM.
Biochemical analyses demonstrated that GSK2593074A was a type II kinase inhibitor. GSK2593074A bound to and inhibited both RIP1 and RIP3. In multiple cell types including mouse smooth muscle cells, fibroblasts, bone marrow derived macrophages, and human colon epithelial cells, GSK2593074A inhibited necroptosis with an IC50 of ~3 nM. Furthermore, GSK2593074A was well tolerated by mice. GSK2593074A attenuated vascular inflammation and aortic expansion in two distinct abdominal aortic aneurysm models and in both male and female mice.
In summary, the high potency and minimum cytotoxicity make GSK2593074A a desirable drug candidate of pharmacological therapies.
Reference:
Cell Death Dis. 2019 Mar 6;10(3):226.