The 5-lipoxygenase (LOX) pathway plays an important role in the pathophysiology of asthma. 5-LOX is an important arachidonic acid-metabolizing enzyme producing leukotrienes and other proinflammatory lipid mediators. Moreover, it has potent pathophysiological functions in asthma and other inflammatory diseases. The production of leukotrienes C4, D4, and E4 requires 5-LOX. They are potent bronchoconstrictors and proinflammatory mediators. Meanwhile, the 5-LOX enzyme is also involved in the production of LTB4. It is a primary attractant and activator for leukocytes. In addition, 5-LOX activity also results in the production of bioactive metabolites 5-hydroxyeicosatetraenoic acid and 5-oxo-6,8,11,14-eicosatetraenoic acid. PF-4191834 is a selective non-redox 5-lipoxygenase inhibitor effective in inflammation and pain.
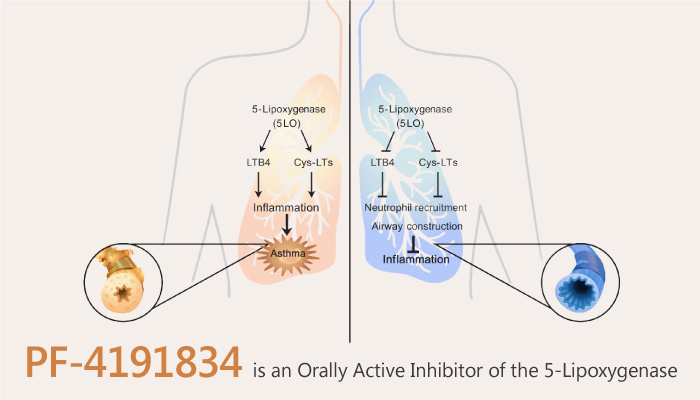
PF-4191834 is a selective non-redox 5-lipoxygenase inhibitor effective in inflammation and pain.
In vitro, PF-4191834 inhibits 5-LOX with an IC50 value of 229 nM. In addition, PF-4191834 is a non-redox inhibitor. It exerts a concentration-dependent inhibition of human 5-LOX, with an IC50 value of 130 nM and an IC80 value of 370 nM. Moverover, it completely inhibits the synthesis of the 5-LOX products 5-HETE, 5-oxo-ETE, LTB4, and LTE4 with estimated IC50 values between 100 and 190 nM.
In vivo, oral administration of PF-4191834 results in a concentration-dependent inhibition of pouch fluid levels of LTB4, with ED50 and ED80 values of 0.46 and 0.93 mg/kg, respectively. On the other hand, there is a similar dose-response inhibition in an ex vivo rat blood assay. It has ED50 and ED80 values of 0.55 and 1.3 mg/kg, respectively. Furthermore, at the 3 and 10 mg/kg dosages, PF-4191834 reduced LTB4 concentration in the paw exudates by 85 and 90%, respectively.
All in all, PF-4191834 has the potential to realize the full benefits of 5-LOX inhibition. PF-4191834 is a noniron chelating, non-redox inhibitor of the 5-LOX enzyme that is being developed as an oral anti-inflammatory therapy for the treatment of asthma.
Reference:
Masferrer JL, et al. J Pharmacol Exp Ther. 2010 Jul;334(1):294-301.