Hepcidin is a key player in iron metabolism and its role as an iron regulatory hormone. Hepcidins exhibit antifungal activity against Candida albicans, Aspergillus fumigates, and Aspergillus niger and antibacterial activity against Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis. Especially, Hepcidin is the central regulator of systemic iron homeostasis. Dysregulation of hepcidin production results in a variety of iron disorders. Hepcidin deficiency is the cause of iron overload in hereditary hemochromatosis, iron-loading anemias, and hepatitis C. Moreover, Hepcidin lowers serum iron concentrations by counteracting the function of ferroportin. Usually, Hepcidin exerts its effects through its receptor, the cellular iron exporter ferroportin. Hepcidin also controls iron release from cells that recycle or store iron, thus regulating plasma iron concentrations. This study leads to the design of a potent and bioavailable hepcidin production inhibitor DS28120313.
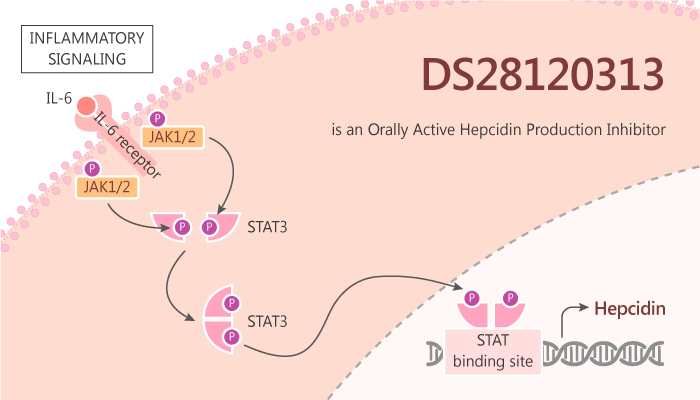
In this study, TakeshiFukuda, et al report the design and synthesis of DS28120313. Particularly, DS28120313 inhibits hepcidin production in HepG2 cells. Furthermore, DS28120313 has a suitable profile as an oral agent. DS28120313 shows serum hepcidin-lowering effects in an interleukin-6-induced acute inflammatory mouse model. The appropriately lipophilic compound DS28120313 possesses good metabolic stability and low plasma protein binding. Further, DS28120313 shows high plasma exposure in mice. In particular, DS28120313 significantly reduces the blood hepcidin level at an oral dose of 30 mg/kg in an IL-6-induced acute inflammatory mouse model.
All in all, the peptide hormone hepcidin plays a central role in regulating dietary iron absorption and body iron distribution. Subsequently, many human diseases are associated with alterations in hepcidin concentrations. DS28120313 is a potent orally available hepcidin production inhibitor.