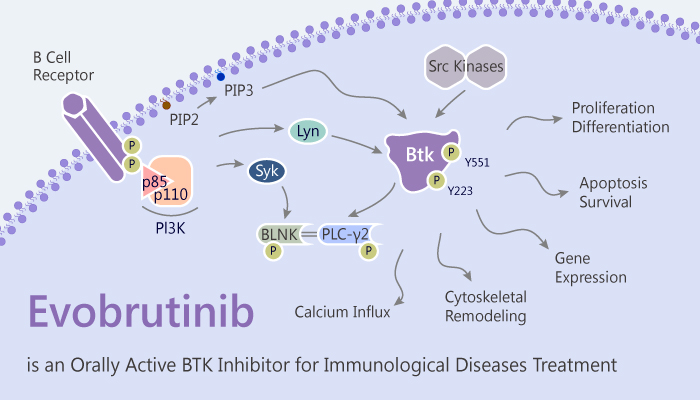
Bruton’s tyrosine kinase (BTK), a member of the Tec family of tyrosine kinases, is expressed in B cells, macrophages, and monocytes, but not in T cells. Mutations in the human Btk gene can result in the B cell specific immunodeficiency, X-linked agammaglobulinemia,; this leads to a lack of B cells and plasma cells, and markedly reduced levels of serum immunoglobulins. Consequently, BTK represents as a promising therapeutic target for the treatment of immunological diseases.
For instance, Among the FDA approved clinical drugs, Ibrutinib is an irreversible BTK inhibitor, covalently targets Cys481 within the ATP-binding pocket. To illustrate, it has been applied in clinical for the treatment of mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, Waldenström’s macroglobulinemia, and marginal zone lymphoma, as well as graft versus host disease. However, the first approved BTK inhibitor also showed side effects, including bleeding, rash, diarrhea, and atrial fibrillation. And the side effectsresulted from, in part, to off-target kinase-inhibitory related effects on other Tec family kinases.
Additionally, a study from Richard Caldwell identified Evobrutinib (M2951) as an orally active, potent, highly selective and irreversibly covalent BTK inhibitor. It exhibits an IC50 of 8.9 nM.
The authors verified the activity of Evobrutinib for related disease in female lewis rats with semi-established type II collagen arthritis, Evobrutinib (0.3, 1, 3, 10, or 30 mg/kg, oral gavage, once daily) demonstrates efficacy in a rat model of rheumatoid arthritis.
Typically, compared to Ibrutinib, Evobrutinib exhibits highly selectivity for BTK over EGFR and other Tec family kinases. The selectivity suggested a low potential for off-target related adverse effects. The conclusion suggested that Evobrutinib may has less side effects.