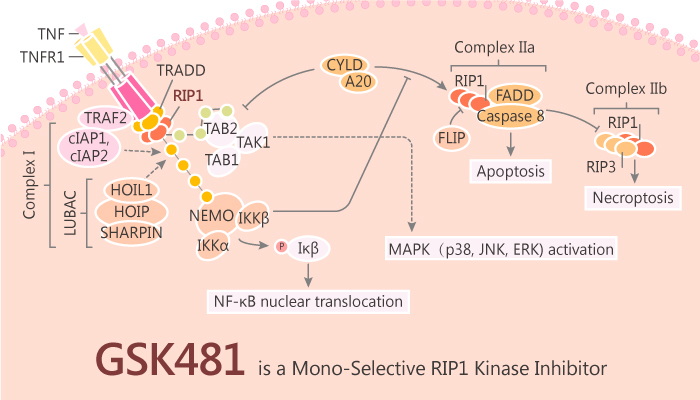
Recent research shows that RIP1 kinase activity is an important driver of TNF-mediated inflammation and pathology. Genetic evidence of mice supports it. The mutations shunt signaling down the RIP1 kinase pathway and result in spontaneous and robust inflammation. RIP1 has now revealed that activated RIP1 can also directly regulate pro-inflammatory cytokine production and some forms of apoptosis. Furthermore, in addition to its role downstream of TNF receptor 1 (TNFR1), RIP1 is also a critical driver of inflammation downstream of various other pathways. Thus, inhibition of RIP1 activation is likely to have a broad therapeutic potential for multiple inflammatory diseases. In this study, RIP1 against GSK’s DNA-encoded small-molecule libraries and identifies a highly potent benzoxazepinone inhibitor series. GSK’481 is a potent, selective, and specific RIP1 inhibitor.
Upon profiling in the RIP1 assays, GSK’481 shows an increase in biochemical activity. It also exhibits excellent translation in the U937 cellular assay (IC50=10 nM). GSK’481 shows complete specificity for RIP1 kinase over all other kinases. At a concentration of 10 μM, GSK’481 represents an estimated >7500-fold selectivity window based on the RIP1 ADP-Glo potency of 1.3 nM. GSK’481 exhibits approximately equivalent RIP1 FP potencies against human and cynomolgus monkey RIP1. But, it is >100-fold less potent against nonprimate RIP1.
On the other hand, GSK’481 is a potent inhibitor of S166 phosphorylation in wild-type human RIP1 (IC50=2.8 nM). However, it is ineffective at reducing S166 phosphorylation for wild-type mouse RIP1 (IC50>10 μM). GSK’481 inhibits Ser166 phosphorylation in all three mouse RIP1 mutants more potently than in wild-type mice.
In summary, GSK’481 is an excellent starting point for further optimization into a RIP1 clinical candidate.
Reference:
Harris PA et al. J Med Chem, 2016 Mar 10, 59(5):2163-78.