Chronic hepatitis C (CHC) virus infection continues to be a significant health burden, and 2–3% of the global population infected by the hepatitis C virus (HCV). The innate immune response is critical for the eradication of HCV in vivo, with type 1 interferons (IFNs) stimulating many genes with antiviral properties. IFN-α is just one component of this response. In addition, Toll-like receptor 7 (TLR7) is a member of the Toll-like receptor family. It is a group of receptors that recognize pathogen-associated molecular patterns. Besides, recognition of viral single-stranded RNA by TLR7 leads to the initiation of a host antiviral response. Additionally, TLR7 stimulation generates a significant proinflammatory response. It has the potential risk of side effects similar to those seen after exogenous treatment with Peg IFN‐α. PF-4878691 is a potent, orally active, and selective Toll-like receptor 7 (TLR7) agonist modeled to dissociate its antiviral and inflammatory activities.
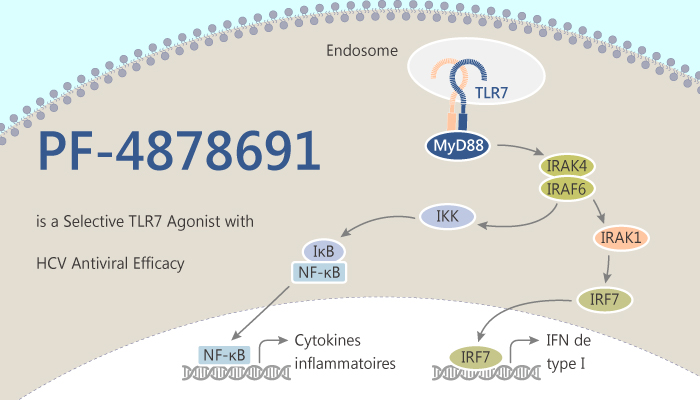
PF-4878691 induces an antiviral response equivalent to that seen with Peg-IFN-α treatment, at doses associated with limited AEs. Moreover, PF-4878691 induces biomarkers of the immune and IFN responses in a dose-dependent and dose-frequency-related manner. However, two subjects in the top (9 mg) dose group experienced serious adverse events, characterized by flu-like symptoms, hypotension, and lymphopenia, leading to termination of the study and further clinical development of the compound.
In summary, PF-4878691 is a potent, orally active, and selective Toll-like receptor 7 (TLR7) agonist. TLR7 stimulation results in a pharmacologic response at levels commensurate with predicted antiviral efficacy. But, serious adverse events have a relation with these doses, raising concerns about the therapeutic window of this class of compounds for the treatment of HCV infection.
Reference:
Fidock MD, et al. Clin Pharmacol Ther. 2011 Jun;89(6):821-9.