Recruitment of myeloid-derived suppressor cells (MDSCs) into tumors induces local immunosuppression in carcinomas. CXCR2+ neutrophilic MDSCs (PMN-MDSCs) are the most abundant myeloid cell subset within oral and lung syngeneic carcinomas. Meanwhile, PMN-MDSCs demonstrated greater suppression of tumor-infiltrating lymphocyte killing of targets compared with macrophages. Depletion or pharmacologic inhibition of MDSCs enhances responses to PD-axis immune checkpoint blockade in syngeneic models of SCC and adenocarcinoma. Strategies to deplete MDSCs are not easily translated clinically, and small-molecule inhibitors blocking MDSC function may alter the function of effector immune cells. In this study, selective expansion and tumor trafficking of CXCR2+ PMN-MDSCs suppress the effector function of antigen-specific tumor-infiltrating lymphocytes (TILs). SX-682 is a novel small-molecule allosteric inhibitor of CXCR1 and CXCR2. Moreover, it abrogates tumor accumulation of PMN-MDSCs and enhances the efficacy of both PD-axis immune checkpoint blockade and adoptive cell transfer of engineered T cells.
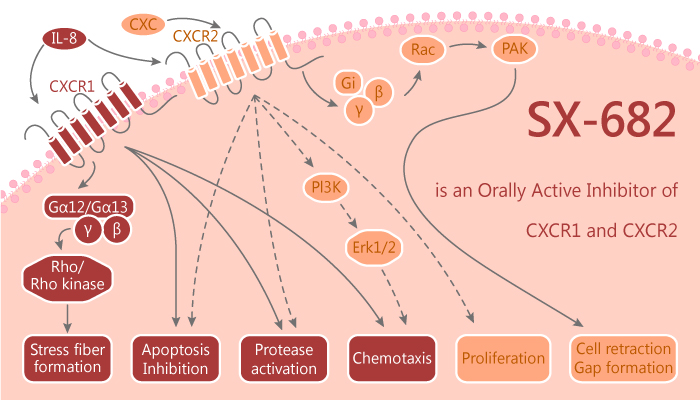
SX-682 is an orally bioavailable small-molecule inhibitor of CXCR1 and CXCR2. SX-682 monotherapy abrogates CXCR2+ PMN-MDSC tumor infiltration. Furthermore, it enhances the antitumor efficacy of the PD-axis immune checkpoint blockade. In addition, abrogation of PMN-MDSC tumor infiltration with SX-682 can enhance the therapeutic efficacy of adoptively transferred T cells. The enhanced therapeutic efficacy of PD-axis immune checkpoint blockade and OT-I adoptive cell transfer following SX-682 treatment is primarily due to reduced tumor infiltration of immunosuppressive CXCR2+ PMN-MDSCs. It is not due to reduced CXCR2 expression, abrogated PMN-MDSC suppressive capacity, or direct antitumor cell effects.
All in all, SX-682 to enhance responses to PD-axis immune checkpoint blockade and adoptive transfer of engineered T cells through selective inhibition of CXCR2+ PMN-MDSC trafficking into tumors. Tumor PMN-MDSCs may be important modulators of response to both forms of immunotherapy.
Reference:
Sun L, et al. JCI Insight. 2019 Apr 4;4(7).