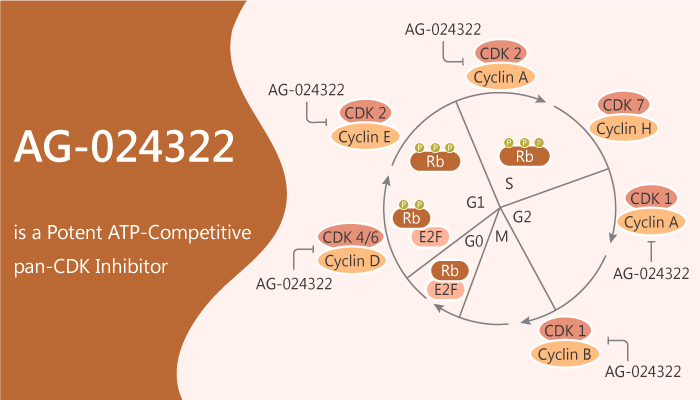
Mammalian cell division involves an orderly progression through four distinct phases: G1 (growth phase 1), S (DNA synthesis), G2 (growth phase 2), and M (mitosis). During the G1 phase, cells respond to extracellular signals by moving towards cell division or entering into a quiescent state (G0). And then Cyclin D-CDK4, cyclin D-CDK6, and cyclin E-CDK2 protein contribute to the G1 to S phase transition. lastly, CDK2 and cyclin B-CDK1 protein relate to the S-phase and G2–M transition, respectively.
Cyclin-dependent kinases (CDKs) play an important role in the control of cell-cycle progression. Additionally, they also exhibit aberrant regulation in various neoplastic diseases.
In this article, we will introduce a potent ATP-competitive pan-CDK inhibitor, AG-024322.
Firstly, in vitro, in human PBMCs, AG-024322is less toxic at concentrations below 3 µM. The viability of human PBMCs as measured by ATP content with a TC50 value of 1.4 µM. Furthermore, AG-024322 exhibits growth inhibition effects on HCT-116 cells. This inhibitor shows less potent in the functional cellular assay with an IC50 of 120 nM.
Nextly, in vivo, Male and female cynomolgus monkeys are intravenous infusion with a varied dosage of AG-024322 for 5 days. As a result, AG-024322 exhibits no-adverse-effect at 2 mg/kg with mean plasma AUC(0-24.5) of 2.11 g.h/mL. At 6 mg/kg produces pancreatic bone marrow hypocellularity, lymphoid depletion. When the dosage is at 10 mg/kg, vascular injury at the injection site renal tubular degeneration occurs.
In human tumor mice xenografts of different origins, AG-024322 inhibits the growth of tumor growth inhibition (TGI) ranging from 32% to 86.4%. It also demonstrates anti-tumor effects as a dose-dependent manner. Lastly, when the dosage for mice reaches 20 mg/kg.
In the MV522 tumor model, AG-024322 causes a 65% TGI. It results in a 52% TGI at 1/2 of the maximum tolerated dose (MTD) and only slight anti-tumor activity at 1/4 of the MTD.
In conclusion, as a potent inhibitor of CDK1, CDK2, and CDK4, AG024322 produces cell-cycle arrest in vitro. Whatmore, it exhibits antitumor activity in both money and mice model.
Reference:
[1]. Brown AP, et al. 2008 Nov;62(6):1091-101.
[2]. Cathy C. Zhang, et al. Cellular and Molecular Biology 53: Cell Cycle Control and Cancer 1