Multiple sclerosis (MS) is an immune-mediated neurodegenerative disease of the central nervous system (CNS) and is a leading cause of chronic neurological disability.
The most remarkable hallmarks of MS are neuroinflammation and closely associates with axonal injury. During the MS pathological process of MS, the infiltration of myelin-reactive T cells into the CNS and leads to the disruption of the communication of the nervous system.
The pathogenesis of MS remains elusive. However, emerging evidence supports that caspase-1, interleukin IL-1β, and IL-18 plays an important role in the pathogenesis of MS. Moreover, activation of caspase-1 and pro- IL-1β slicing is closely related to the NLRP3 inflammasome.
The severity of MS and IL-1β has a close correlation. Furthermore, IFN-β is an effective method for MS treatment and is NLRP3 inflammasome-dependent. As a result, NLRP3 is a potential target for research.
In this article, we will introduce a selective NLRP3 inflammasome inhibitor, JC-171.
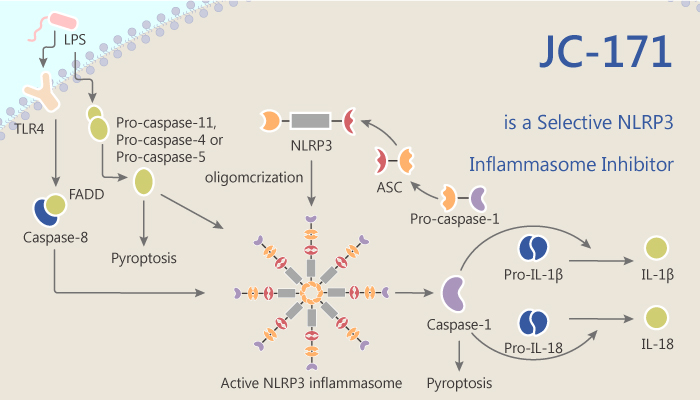
It blocks NLRP3 inflammasome activation and IL-1β production in primary macrophages dose-dependently. The IC50 for inhibiting LPS/ATP-induced interleukin-1β release from J774A.1 macrophages is of 8.45 μM.
In mice injected with MOG35-55 peptide, JC-171 treatment delays the progression and reduces the severity of experimental autoimmune encephalomyelitis (EAE) in the mouse. Meanwhile, it results in a substantial decrease in the frequency of MOG35-55-specific Th17 cells in the spleens and spinal cords of EAE mice.
From all the above, in vitro, JC-171 is a selective inhibitor of the NLRP3 inflammasome and inhibits IL-1β release from macrophages. Besides, the inhibition potency of JC-171 is comparable to the parent compound JC-21. The result demonstrates that sulfonamide modifications based on the parent structure are well tolerated. In vivo JC-171 administration effectively blocks disease progression in EAE mice.
In conclusion, JC-171 is a promising NLRP3 inflammasome inhibitor and plays an important role in vivo and in vitro.
Reference:
Chunqing Guo, et al. ACS Chem Neurosci. 2017 Oct 18;8(10):2194-2201.