The AAA+ ATPase p97 is essential to a wide range of cellular functions. The p97 acts downstream from ubiquitin signaling events and utilizes the energy from ATP hydrolysis to extract its substrate proteins from cellular structures or multiprotein complexes. Ubiquitin-interacting domains and p97-binding domains combine to form bi-functional cofactors. In addition, p97 inhibition acts as a promising approach to provoke proteotoxic stress in tumors. CB-5083 is a selective and orally bioavailable p97 inhibitor.
CB-5083, a first-in-class inhibitor of p97, has broad antitumor activity in a range of both hematologic and solid tumor models. CB‐5083 is well‐tolerated in mice, it would be important to further develop this agent for additional preclinical and clinical studies. Furthermore, CB-5083 has robust activity against multiple myeloma cell lines and a number of in vivo multiple myeloma models. Single-agent CB-5083 inhibits tumor growth and combines well with multiple myeloma standard-of-care agents.
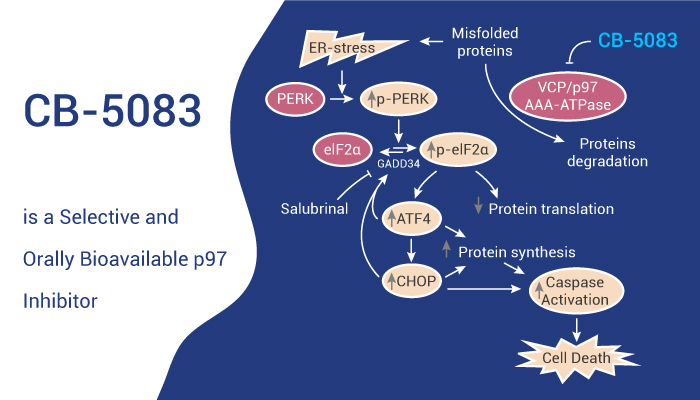
CB-5083 is a potent and highly selective inhibitor of the D2 ATPase domain of p97. CB-5083 decreases viability in multiple myeloma cell lines and patient-derived multiple myeloma cells. Moreover, CB-5083 has a unique mechanism of action that combines well with proteasome inhibitors. Treatment with CB-5083 is associated with accumulation of ubiquitinated proteins, induction of the unfolded protein response, and apoptosis. In addition, CB-5083 disrupts protein homeostasis upstream of the proteasome.
All in all, CB-5083 is the first inhibitor of p97 to demonstrate significant tumor growth inhibition in vitro and vivo. Inhibition of p97 by CB-5083 activates the unfolded protein response and subsequently induces apoptosis in a wide variety of hematologic and solid tumor cell lines.