Phospholipases A2 (PLA2s) constitute a superfamily of enzymes that catalyze the hydrolysis of fatty acids from the sn-2 position of glycerophospholipids to produce free fatty acids and lysophospholipids. However, free fatty acids and lysophospholipids are both involved in signaling transduction and metabolic processes, and account for a vast number of diseases. Of all the PLA2s, lipoprotein associated phospholipase A2 (Lp-PLA2) in particular has gained increasing attention as a growing number of epidemiological and experimental studies suggest that it plays an important role in diseases such as atherosclerosis, diabetes, and asthma. Furthermore, study has shown a strong, positive correlation between levels of Lp-PLA2 and coronary events. Meanwhile, they showed that Lp-PLA2 is a new, independentmarker of coronary heartdisease risk. Therefore, PLA2s are obvious candidates for pharmacological research and intervention. Hence, we will introduce a potent Lp-PLA2 inhibitor-Darapladib (SB-480848).
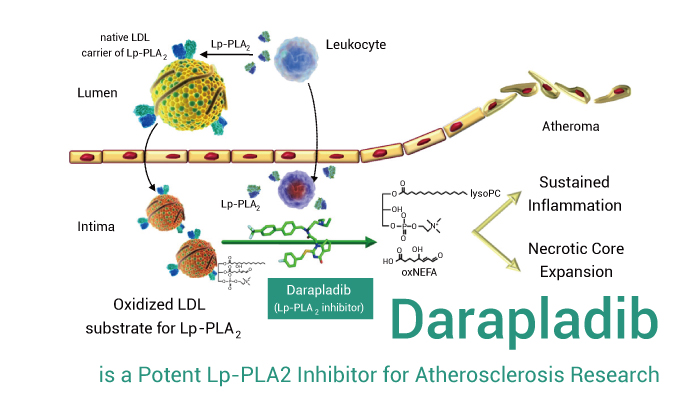
Darapladib is an orally active, selective and reversible Lp-PLA2 inhibitor.
Darapladib can trigger irreversible actions on glioma cell apoptosis and mitochondrial dysfunction. In vitro, Darapladib (5 μM; 6 and 12 h) induces subG1 cell cycle arrest in glioma cells (C6 glioma cells and U251MG cells). Moreover, Darapladib (5 μM; 3 and 6 h) triggers cell apoptosis in glioma cells. Besides, Darapladib (5 μM; 5, 15, 30, 60 and 90 min) induces an increase in phosphorylation of ERK1/2 proteins, but reduces AKT phosphorylation in glioma cells.
Darapladib not only shows good activity in cancer research, but also atherosclerosis. Darapladib (50 mg/kg; p.o.; once daily for 6 weeks) significantly inhibits serum Lp-PLA2 activity in LDLR-deficient mice. In addition, inhibition of Lp-PLA2 by Darapladib decreases serum hs-CRP and IL-6 levels, but has no significant effects on the PAF level.
All in all, Darapladib is a promising Lp-PLA2 inhibitor for research of cancer, atherosclerosis and coronary artery disease.
Reference:
[1] Blackie JA, et al. Bioorg Med Chem Lett. 2003 Mar 24;13(6):1067-70.
[2] Wang YJ, et al. Toxicol Appl Pharmacol. 2020 Sep 1;402:115133.
[3] Hu MM, et al. Acta Pharmacol Sin. 2011 Oct;32(10):1253-8.
[4] Riley RF, et al. IDrugs. 2009 Oct;12(10):648-55.