mGluR (metabotropic glutamate receptor) is a type of glutamate receptor that are active through an indirect metabotropic process. They are members of thegroup C family of G-protein-coupled receptors, or GPCRs. In addition, like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatoryneurotransmitter. The mGluRs perform a variety of functions in the central and peripheral nervous systems: mGluRs are involved in learning, memory, anxiety, and the perception of pain. mGluRs are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues.
Eight different types of mGluRs, labeled mGluR1 to mGluR8, are divided into groups I, II, and III. Receptor types are grouped based on receptor structure and physiological activity. Among them, group II mGluRs (mGluR2, mGluR3) are presynaptic and are autoinhibitory GPCRs such that inhibition of mGluR2 has been shown to increase synaptic glutamate, increase postsynaptic activity and plasticity, and potentially improve learning and memory.
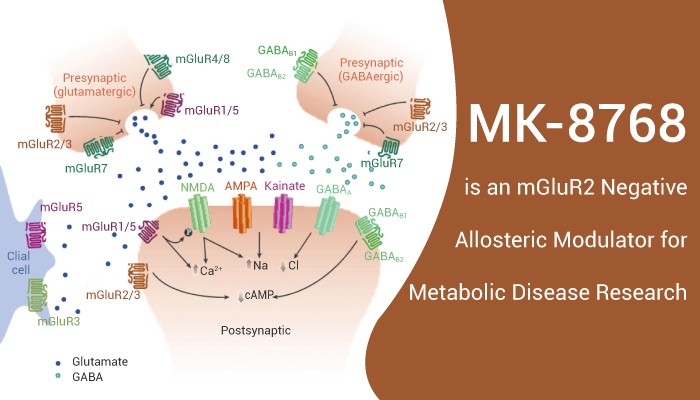
MK-8768 is a highly potent, orally active and selective mGluR2 negative allosteric modulator with excellent brain permeability.
MK-8768 is more selective for mGluR2 than mGluR1, mGluR3, mGluR4, mGluR5, mGluR6, mGluR8 (IC50 >10 000 nM). In addition, MK-8768 also shows an excellent overall selectivity profile with weak to no activity on the hERG, IKs, and Nav1.5 ion channels. Moreover, MK-8768 is a nonsubstrate for rat, human, and monkey P-gp and demonstrates good brain penetration in rats. In rats, dogs, and monkeys, MK-8768 exhibits moderate clearance, and the effective half-life ranged from 1.7 h in monkeys to 3.3 h in rats. Besides, the oral bioavailability is 32% and 34% in rats and dogs, respectively. Furthermore, MK-8768 shows efficacy in a rhesus monkey model of executive function and attention.
To sum up, MK-8768 is a selective, orally active, and brain-penetrant mGluR2 negative allosteric modulator.
References:
[1] Michael T Rudd, et al. ACS Med Chem Lett. 2023 Jul 12;14(8):1088-1094.